1. Introduction
Soft tissue sarcoma (STS) is a rare tumor of mesodermal origin (1). Most of these tumors are sporadic and genetic factors, lymphedema, and post-radiation may be implicated in some patients (2). There are more than 100 different types of histology in sarcoma, which present different clinical behaviors (3). Liposarcoma, leiomyosarcoma, and undifferentiated pleomorphic sarcoma are among the most common pathologies (2). The most common sites of involvement include extremities, trunk, retroperitoneum, as well as head and neck (1). The cornerstone of the treatment in localized cases regardless of histology is surgical resection, with the aim of organ and function preservation with a negative margin (4). Our study presented a rare case with 2 types of sarcomas with undifferentiated pleomorphic sarcoma (UPS) and low-grade epithelioid leiomyosarcoma pathologies synchronously.
2. Case Presentation
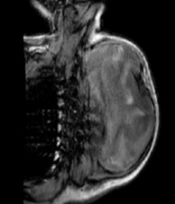
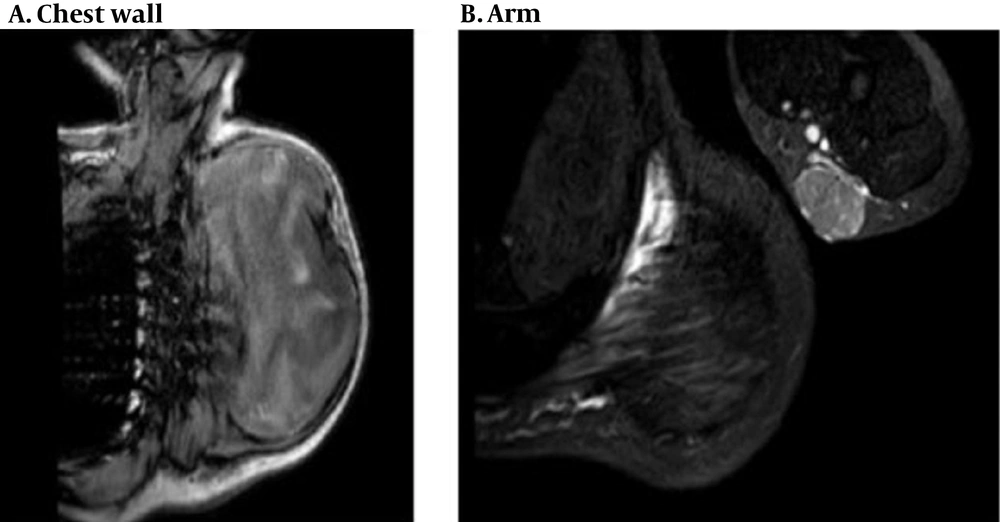
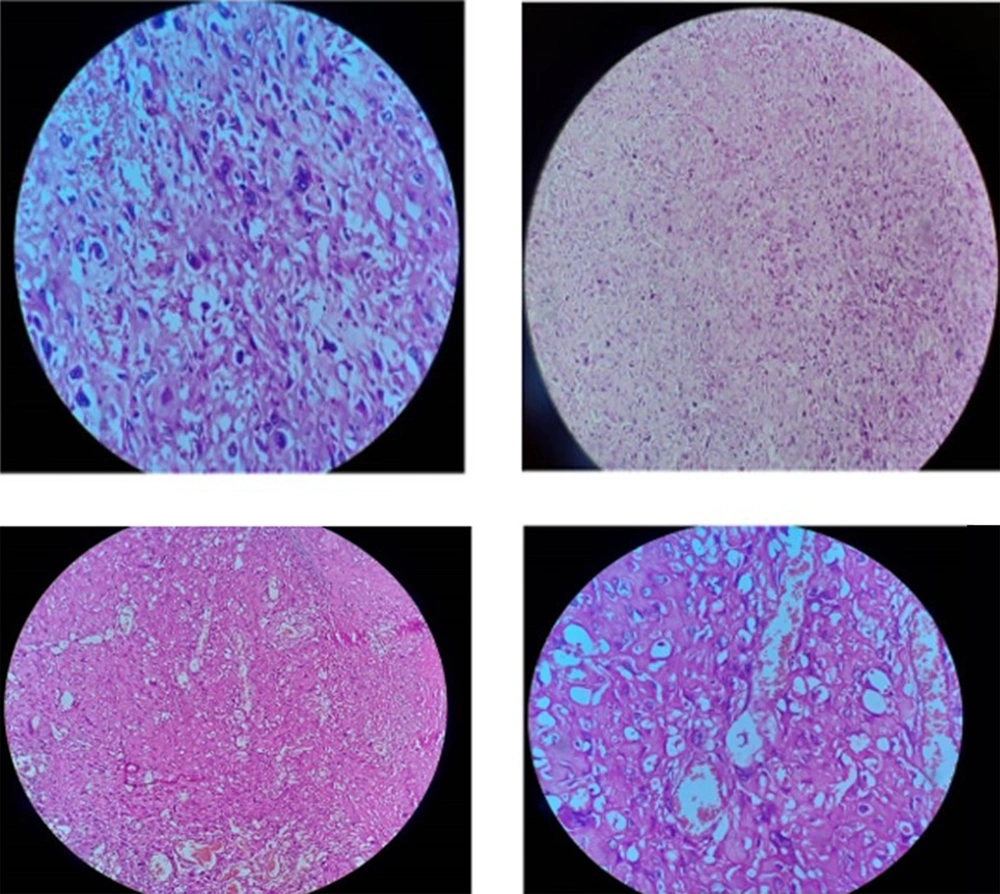
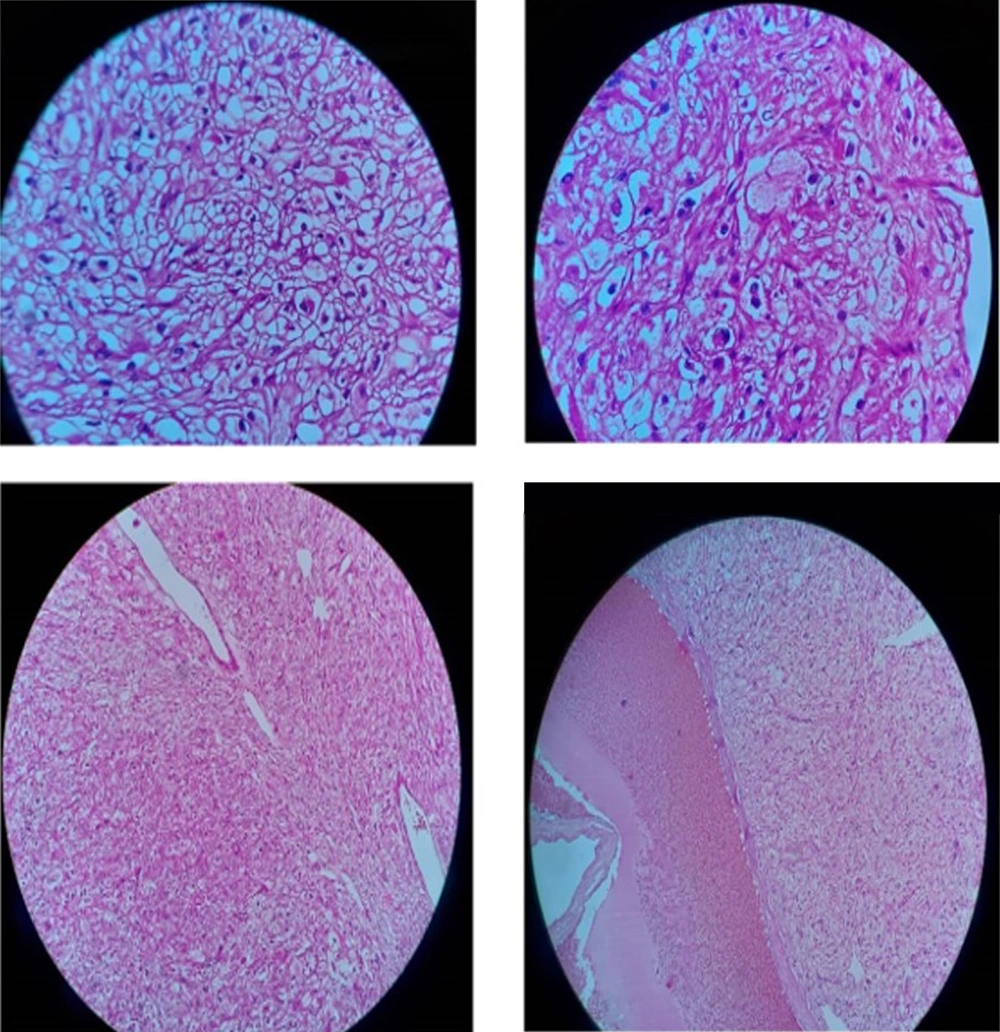
The patient was a healthy 46-year-old man with a progressive mass in the right posterior of the chest wall and a slow-growing mass in his arm. There was no tenderness and lymphadenopathy in the physical exam. Core needle biopsy led to the undifferentiated pleomorphic sarcoma. MRI revealed a huge 250 × 187 mm high signal intensity mass in T2W and low signal in T1W deep to the left shoulder muscle from the base of the neck down to the left lateral of the chest wall. A 42 × 29 mm mass in the elbow was also seen (Figure 1). Metastatic workup was negative by exam and CT scan. He received 2 cycles of MAI (mesna, doxorubicin 25 mg/m2 d1-d3 and ifosfamide 2500 mg/m2 d1-d4 every 3 weeks) regimen chemotherapy due to large and multiple masses, but the tumor size did not change. After consultation with an orthopedic oncosurgeon, wide masses resection was done. The pathology of chest wall mass was compatible with UPS with the size of 32 × 20 cm and more than 50% necrosis. The nearest tumor margin was 2 mm (Figure 2). The diagnosis was confirmed by immunohistochemistry (IHC) which was weakly positive for smooth muscle actin (SMA) and Desmin in a few tumor cells. Arm mass histology was suggestive of low-grade epithelioid leiomyosarcoma with smooth muscle differentiation with the size of 6 × 5 cm and 1/mm2 mitotic count (Figure 3); IHC was positive for SMA, Desmin, and h-Caldesmon and negative for Melan-a, S100, SOX10, CD34, and CKAE1/AF3. Adjuvant chemotherapy was continued for a total of 6 courses. The patient received radiation with a 60 Gy total dose to the posterior aspect of the chest wall. According to low-grade histology and negative margins, adjuvant radiation failed to perform for arm mass. No recurrence or complication was observed after 4 months of follow-up.
Microscopic view of the chest wall mass: Hyalinized sclerotic tumoral lesion is seen, intermixed and rimmed by clusters of Bizarre epithelioid atypical cells with severe pleomorphic hyperchromatic nuclei, high N/C ratio, irregular nuclear membrane and some with prominent nucleoli, and pink to clear cytoplasm. Some atypical mitotic figures are seen.
3. Discussion
Sarcomas are classified based on morphology, IHC, and genetic characteristics and have different clinical behaviors (3). The most common sign at the time of diagnosis is painless mass (4). One of the effective factors in the relapse pattern and outcome is the site of disease (2). About 40 - 50% of cases occur in the limbs, and 12% in the trunk (3). Nevertheless, the tendency to early local relapse in retroperitoneal masses is higher (2). The treatment of these patients, considering its rarity, should involve a multidisciplinary team (3). The treatment modalities that are practical in STS include surgery, radiotherapy, and chemotherapy (3). Sometimes and uncommonly, the risk of secondary carcinoma increases in STS patients even in the absence of genetic syndromes (4). However, few patients with multiple soft tissue sarcoma have been reported; these cases are described as follows:
In the review by Lex et al. in examining the literature, they found 17 patients with multiple STS. The average time between the first and the second STS was around 2.3 years, but in 6 patients it existed synchronously (4).
In the study by Grobmyer et al., among 5505 patients with STS from 1982 to 2003, 9 patients with multiple STS were found, which was metachronous in 7 patients; indeed, the prevalence of secondary STS in these patients was 0.2%, which is higher than the normal population (5).
In the study by Merimsky et al., investigating 610 STS patients with bone sarcoma who had been treated in 1995 - 1999, 7.5% (n = 28) of patients had secondary malignancy before or after STS. In 14 patients, STS was the primary tumor, while the secondary tumors included STS (3patients), Renal cell carcinoma (RCC), breast, colon, melanoma, Non-small-cell carcinoma ( NSCLC), prostate, and papillary thyroid carcinoma ( PTC) (6).
In 1980, Schiffman reported a synovial sarcoma case in the knee followed by epithelial sarcoma with lymphatic metastasis in the contralateral knee (7).
In 2014, Scepanovic et al. reported a 19-year-old patient with bilateral shoulder Malignant fibrohistocytoma (MFH) as symmetric and metachronous with a 1.5-year interval (8).
The most common method for tumor response evaluation after chemotherapy is response evaluation criteria in the solid tumor (RECIST) criteria but in soft tissue sarcoma, functional imaging is superior (9). In our case, the size of the tumor did not change by chemotherapy but there was more than 50% necrosis which is representing tumor response.
3.1. Conclusions
According to previous reports and this report, multiple STS is rare; nevertheless, its probability, either synchronous or metachronous, should be considered during patient examine and follow-up. In the case of a secondary lesion, different histology is probable, and the patient should be biopsied and imaged.