1. Background
Colorectal cancer (CRC) is one of the most common and deadly neoplasms (malignancies) of the gastrointestinal tract. CRC has been reported as the third most prevalent cancer, the fourth cancer-related cause of death, worldwide, and the second cause of death in Europe (1, 2). More than 1.4 million new cases of CRC (almost 9.7% of all cancers) are annually identified and more than half a million of whom die (1). The incidence distribution of CRC is geographically and racially quite diverse in different countries. Different geographical distributions are known to have been caused by diet (3, 4). Research suggests an annual incidence and mortality of respectively more than 3641 and around 2263 cases in Iran (5).
CRC is a disease that starts from epithelial cells in the large intestinal and causes the growth of tumors. CRC occurs commonly in the appendix, colon, and rectum (6). In fact, this neoplasm occurs as a result of progressive accumulation of genetic and epigenetic variations with increasing age as well as with the presence of pre-disposing risk factors, and causes adenoma and ultimately adenocarcinoma through pathological changes in the normal epithelium of the large intestine (7, 8).
The risk factors contributing to CRC include increasing age, genetics, and lifestyle, i.e. diet, immobility, obesity, and diabetes (9). Diet plays a key role in preventing the cancer as many studies have demonstrated the relationship between nutritional factors and CRC. High-fiber and low-fat diets, physical activity as well as absence of smoking and alcohol consumption reduce the risk of CRC (10, 11). On the contrary, high-fat, high-calorie and low-fiber diets, limited physical activity as well as smoking and alcohol consumption prolong the transit and handling time of food through the epithelial cells (4, 11). As a result, colorectal epithelium cells will be more exposed to the effects of mutagenic compounds, thereby, increase the risk of CRC (12).
The aggressively metastatic behavior, as the most important aspect in patients with cancer, is mainly caused by changes in molecular characteristics of tumoral cells including disruption of cell growth and proliferation control (13). Different biomarkers associated with oncogenes and tumor suppressor genes, growth factors, angiogenesis, and cell proliferation factors are used in the diagnosis and prognosis of the cancer. The nuclear antigen, Ki67, is a non-histone protein, which is present in all cell cycle phases except in the phase G0 and plays a key role in cell proliferation (14). Owing to its high sensitivity, Ki67 is extensively used to assess the proliferation of cancer cells (15, 16).
Most of the studies using IHC employed Ki67 as an indicator in the prognosis of well-known malignancies, such as gastric cancer, cancers of gastrointestinal tract, prostate, and breast cancer (15, 17-20). The human epidermal growth factor receptor 2 (HER2) is another prognostic biomarker that is used for detecting tumoral tissues (21). This protein is a transmembrane receptor that is usually present on all normal cells and plays a crucial role in biological activities, such as cell proliferation and survival, differentiation, movement, transformation, inhibition of apoptosis, and even tumor progression (22, 23). Various studies have investigated the HER2 protein role in malignancies of different tissues as well as the importance of its IHC expression in prognosis, response to treatment, and patient’s survival (21, 24).
According to world health organization (WHO), the limitation of detection methods in the early stages of the disease is effective factor on high rates of cancer deaths in developing countries (1). Key decisions in current cancer treatments are subject to acquiring detailed information about the prognosis. As, the correct choice of treatment methods is based on prognostic markers.
This study aimed to investigate the immunohistochemical expression of Ki67 and HER2 in colorectal cancer compared to adenomatous and normal samples.
2. Methods
2.1. Study Design and Sampling
This case-control study was conducted on 137 paraffin-embedded tissue blocks collected from the colorectal tissues in Zahedan, southeastern Iran. All these blocks were selected through convenience sampling method based on inclusion and exclusion criteria from the archive of the pathology department of Ali-ebne-abi-Taleb referral Hospital from 2010 to 2015. The selected colorectal blocks according to the histopathological diagnosis were classified into 3 groups; normal (n = 36), adenomatous (n = 38), and adenocarcinoma (n = 63). All samples were re-evaluated for histopathological confirmation by an expert pathologist before conducting IHC staining.
The inclusion criteria of the specimens were suitable formalin fixed paraffin-embedded tissue along with complete clinicopathological data. The tissues with autolysis specimens, metachronous CRC, inadequate biopsy sample, inflammatory lesions, and other malignancies of gastrointestinal tract were excluded from the study (25). Accordingly, 22 samples were excluded and 137 tissue samples were enrolled to the present study.
2.2. Ethical Considerations
This study was approved by the ethics committee of Zahedan University of Medical Sciences (No: IR.ZAUMS.REC.1394-0327).
2.3. Clinicopathological Assessment
The clinicopathological features of all tissue blocks including age, gender, tumor type, tumor location, cell differentiation, lymph node involvement, and metastasis were assessed.
2.4. Immunohistochemical Staining Procedure
Immunohistochemistry (IHC) staining was performed according to the manufacturer’s instructions. Thin tissue sections (3 µm) were cut, using a microtome (Erma, Japan) from the formalin-fixed paraffin-embedded blocks from colorectal area. The sections were placed on histogrip (CEDARLANE, Canada) coated slides. The tissue sections were, then, deparaffinized in Xylene (3 times × 5 minutes) and rehydrated with descending degree of ethanol (2 times × 5 minutes). Antigen retrieval was performed by placing the container of the slides in 10mM sodium citrate buffer solution (pH = 6) for 20 minutes at 120°C in an autoclave. The slides were, then, cooled at room temperature and deionized in Tris buffer saline (TBS). In order to stop the activity of endogenous peroxide enzymes in this step, the samples were incubated in the presence of hydrogen peroxidase solution 5% and, then, rinsed in TBS. They were placed in the vicinity of proteins blocks (5 minutes) and TBS 2 times, 5 minutes each time. Rabbit monoclonal Ki67 antibody (Novocastra, RTU-Ki67-MM1, England) or HER2 antibody (Novocastra, RTU-HER2-3B5, England) was, then, added to the slides, incubated overnight at 4°C, and rinsed in TBS. In this step, the samples were impregnated in the post-primary block solution for 30 minutes and rinsed in TBS. The Nova Link Polymer solution (30 minutes) was added and again rinsed in TBS. Then, the substrate solution containing Diaminobezidine Chromogen (DAB) was used for 15 to 45 minutes and the slides were, then, washed in distilled water. Mayer’s hematoxylin was used for 3 to 5 minutes to counterstain. The slides were dehydrated, using ethanol 96% and rinsed in Xylene. Plates were finally pasted on the sections, using the glue (Entellan, Germany). Then, 2 expert histologists unaware of clinical diagnosis of the samples evaluated and scored the slides.
The appendix and breast cancer were used as positive control samples for Ki67 and HER2, respectively. In negative control samples, TBS was used instead of a special antibody.
2.5. Ki67 IHC Scoring Method
The samples were scored based on the intensity and extent of nuclei immunostaining (the percentage of positive nuclei), as follows, and the expected immunostaining, according to the protocol, Ki67 was expressed as positive. The immunoreactivity was assessed as defined in the previous studies (26, 27). Immunoreactivity intensity scores: negative (0), mild (1), moderate (2), and strong or intense (3). Immunoreactivity extent: less than 5% immunoreactivity of cells (0), 6% to 25% (1), 26% to 50% (2), 51% to 75% (3), 75% to 100% (4). In order to obtain the final score for each sample, results were calculated by multiplying the immunostaining intensity by percentage of positive cells and reported as 4 groups: negative expression (-, 0), weak positive expression (+, 1 - 3), moderate positive expression (++, 4 - 7), and strongly positive expression (+++, 8 - 12) (20, 28).
2.6. HER2 IHC Scoring Method
The immunoreactivity of HER2 protein was assessed according to previous studies (29, 30), as defined: 0 = no staining or cytoplasmic or membrane staining in < 10% of tumor cells; 1+ = incomplete cytoplasmic or membrane staining in > 10% of tumor cells; 2+ = weak-to-moderate complete membrane or cytoplasmic staining in > 10% of tumor cells; 3+ = moderate-to-strong complete membrane staining in > 10% of tumor cells. Samples with 0 and 1+ score were considered negative expression (normal expression), and 2+ and 3+ score for each slide were classified as positive expression (overexpression).
2.7. Statistical Analysis
Data were represented as mean ± standard error of mean (SEM) and to analysis statistical differences between groups Kruskal-Wallis test was used. Pearson Chi-square or Fisher exact test was used to detect association between histopathological features and Ki67 and HER2 expression status. SPSS 16 under Windows (SPSS Inc., Chicago, IL, USA) was used to analyze the data. P values less than 0.05 were considered significant.
3. Results
A total of 137 tissues were enrolled in the present study. These specimens were from 71 (51.80%) male and 66 (48.20%) female patients with mean age of 48.58 ± 1.49 (age range 13-83 years). The majority of CRC cases were from mucinous adenocarcinoma type 48 (76.20%), in sigmoid region 27 (42.90%), well differentiated 40 (63.50%), and distant metastasis 49 (77.80%). More clinicopathological details were presented in Table 1.
| Colorectal cancer (CRC) | Value |
|---|---|
| Age, mean, y | 57.08 ± 1.76 |
| Gender | |
| Male | 21 (33.30) |
| Female | 42 (66.70) |
| Type | |
| Mucinous adenocarcinoma | 48 (76.20) |
| Non-mucinous adenocarcinoma | 15 (23.80) |
| Location involvement | |
| Cecum | 8 (12.70) |
| Ascending colon | 4 (6.30) |
| Transverse colon | 6 (9.50) |
| Descending colon | 3 (4.80) |
| Sigmoid | 27 (42.90) |
| Anorectal | 15 (23.80) |
| Histological cell differentiation, grade | |
| Well | 40 (63.50) |
| Moderate | 15 (23.80) |
| Poor | 8 (12.70) |
| Metastasis | |
| Yes | 49 (77.80) |
| No | 14 (22.20) |
| Lymph node involvement | |
| Yes | 16 (25.40) |
| No | 47 (74.60) |
| Total | 63 (100) |
| Adenomatous (ADM) | |
| Age, mean, y | 47.71 ± 2.83 |
| Gender | |
| Male | 27 (71.10) |
| Female | 11 (28.90) |
| Location | |
| Cecum | 1 (2.60) |
| Ascending colon | 3 (7.90) |
| Transverse colon | 2 (5.30) |
| Descending colon | 6 (15.80) |
| Sigmoid | 26 (68.40) |
| Total | 38 (100) |
| Normal (N) | |
| Age, mean, y | 34.61 ± 2.27 |
| Gender | |
| Male | 23 (63.90) |
| Female | 13 (36.10) |
| Total | 36 (100) |
aValues are expressed as No. (%).
3.1. Ki67 Clinicopathological Analysis
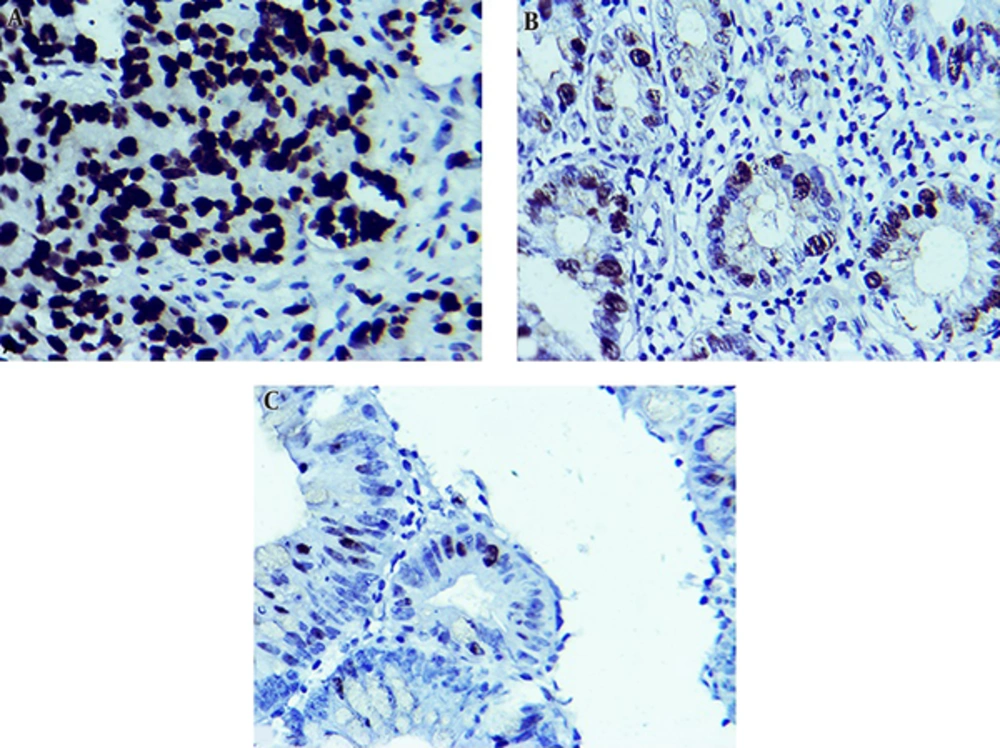
There was not any statistical association between different clinicopathological features of subgroups, including age, gender, tumor type, tumor primary location, lymph node involvement, distant metastasis, and Ki67 overexpression (P > 0.05). But, there was a significant correlation between histological differentiation grade (P = 0.0002) and Ki67 overexpression. Ki67 overexpression in tumors with low differentiation grade was increased (Table 2, Figure 1).
| Index | Tissue Sections | Negative | Positive | Mean ± SEM | ||
|---|---|---|---|---|---|---|
| Weak (Mild) | Moderate | Strong | ||||
| Ki67 | Colorectal cancer (N = 63) | 13 (20.60) | 17 (27.00) | 20 (31.70) | 13 (20.60) | 4.22 ± 0.47 |
| Adenomatous (N = 38) | 21 (55.30) | 12 (31.60) | 5 (13.20) | 0 (0.00) | 1.58 ± 0.11 | |
| Normal (N = 36) | 27 (75.00) | 8 (22.20) | 1 (2.80) | 0 (0.00) | 1.28 ± 0.08 | |
aValues are expressed as No. (%).
3.2. HER2 Clinicopathological Analysis
There was not any statistical correlation between HER2 overexpression and clinicopathological variables, such as age, gender, tumor primary region, histological differentiation grade, and lymph node metastasis (P > 0.05). But, there was a significant association between histological tumor type (P = 0.003) and HER2 positive expression. High expressions of HER2 were observed in tissues with mucinous adenocarcinoma.
The results regarding Ki67 expression in different tissue samples showed that positive expression of Ki67 in CRC, adenomatous, and normal colorectal human tissues were 79.30% (50/63), 44.80% (17/38), and 25% (9/36) respectively. According to Kruskal-Wallis test, there were significant differences regarding Ki67 overexpression between adenomatous and normal tissues (P = 0.0008). This gradually increasing trend of Ki67 positivity was observed from normal to adenomatous tissue and, then, adenocarcinoma.
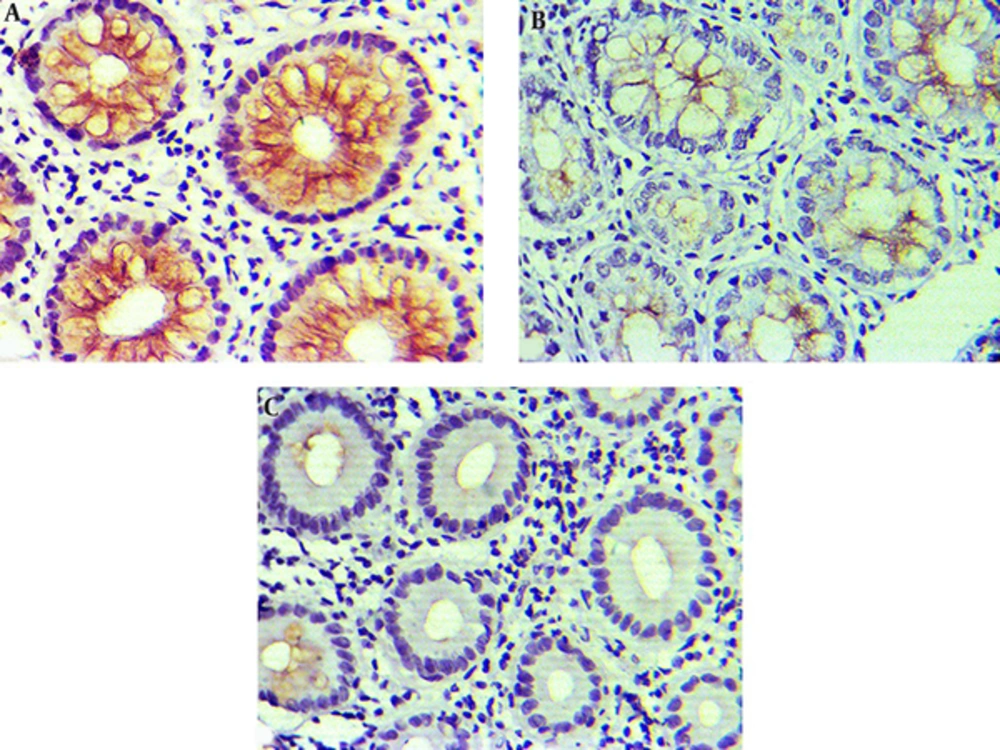
The findings about HER2 status in different tissue samples showed that positive expression in CRC, adenomatous, and normal colorectal tissues were 54.00%, 36.80%, and 19.40%, respectively. Kruskal-Wallis test results showed that there was a significant difference regarding HER2 status between adenocarcinoma, adenomatous, and normal tissues (P = 0.001). HER2 expression was significantly higher in CRC compared to the adenomatous and normal tissues (Table 3, Figure 2).
| Index | Tissue Section | Negative | Positive | Mean ± SEM |
|---|---|---|---|---|
| HER2 | Colorectal cancer (n = 63) | 29 (46.00) | 34 (54.00) | 1.63 ± 0.13 |
| Adenomatous (n = 38) | 24 (63.20) | 14 (36.80) | 1.21 ± 0.17 | |
| Normal (n = 36) | 29 (80.60) | 7 (19.40) | 0.77 ± 0.13 |
aValues are expressed as No. (%).
4. Discussion
The present study indicated that Ki67 expression in colorectal adenocarcinoma was significantly higher than adenomatous and normal tissues. This gradually increasing trend of Ki67 positivity was observed from normal to adenomatous tissue and, then, adenocarcinoma.
CRC is a common malignancy in the gastrointestinal tract, but the mechanisms contributing to its origination, creation, and metastasis is yet unknown. The disruption and imbalance in a series of molecular factors such as onco-protein and tumor suppressor genes is believed to change the healthy tissue to adenomatous and, then, adenocarcinoma (31). The identification and diagnosis of these patients in the early stages, i.e. adenomatous polyps, requires sensitive and efficient methods so that necessary treatments and measures are provided in a timely fashion. Molecular markers can be useful factors in determining the disease progression, prognosis of patient’s condition, and even response to treatments as well as selection of proper therapeutic methods (32).
Ma et al. studied the immunohistochemistry of molecular markers, such as Ki67 in 152 CRC sections and 30 healthy samples and reported higher Ki67 expression in CRC samples compared to healthy samples and a significant difference between nuclear expression of Ki67 in CRC samples and the healthy groups (28). Lin et al. conducted a similar study and found significantly higher Ki67 expression in CRC samples compared to adenoma and normal colorectal tissues (31). Menezes et al. studied 82 patients with colorectal adenocarcinoma and reported positive Ki67 expression in 62 tumoral samples and negative expressions in 20 other samples, indicating significantly high expression of this protein in CRC samples compared to normal colorectal tissues (33). Above-mentioned studies are consistent with the present study in terms of higher expression of Ki67 protein in colorectal adenocarcinoma samples compared to adenomatous and healthy specimens as well as its gradually increment in different phases of the diseases from the healthy tissue to adenomatous polyps and, ultimately, to colorectal adenocarcinoma. Lumachi et al. studied Ki67 expressions, as the prognostic factors in CRC, and found that overexpression of this biomarker was associated with poor prognosis in CRC patients. They also found a significantly inverse correlation between the expression of Ki67 and the survival rate of the patients (34).
Other studies reported relatively contradictory results compared to the present and above-mentioned studies. Salminen et al. reported that the high proliferation activity of Ki67 was associated with increased survival rate of patients with CRC (35). In addition, Melling et al. and Palmqvist et al. found that the low expression of Ki67 index was associated with poor prognosis and metastasis of cancer cells and its higher expression was seen in normal colorectal tissues compared to the CRCs (15, 36). On the other hand, it has been shown in several studies that the high expression of Ki67 was associated with poor prognosis at the higher stages of cancer in prostate and gastrointestinal tumors, endocrine malignancies, and breast cancer (17, 18, 37).
This inconsistency in studies can be attributed to population heterogeneity, wide variation in positively tumoral cells scoring methods, variety in IHC protocol, tumoral tissues with different stages, and even differences in statistical analysis. Nevertheless, a part of these variations probably is due to gene mutation, geographical, and genetically differences that may affect the cell behaviors.
Given that imbalance and disruption in molecular mechanisms that regulate the cell cycle is a cause of cancers (13, 38) and that Ki67 plays a key role in most phases of cell cycles as an important factor in cell proliferation, the expression and proliferation activity of Ki67 seems to increase significantly in cancer cells compared to normal cells.
The present study found no significant association between clinicopathological characteristics such as, age, gender, type of tumor, tumor site, lymph nodes involvement and distant metastasis, and overexpression of Ki67, while there was a significant relationship between cell differentiation grade and Ki67 overexpression. The relationship between clinicopathological features and Ki67 expression seems to be controversial. Melling et al. showed no significant correlation between Ki67 expression with age, gender, tumor site, and histological type tumor, while significant correlation was observed between cell differentiation grade and lymph nodes involvement (15). A study conducted by Hashimoto et al. on the immunohistochemistry of healthy and adenomatous samples and colorectal adenocarcinoma reported that there was no correlation between Ki67 expression and gender, lymph nodes involvement, tumor site, and metastasis, which is consistent with our study (39). Based on the results reported in other studies, Ki67 overexpression was also found to be associated with lymph nodes involvement and high histological grade in CRC patients (40, 41).
On the other hand, Menezes et al. reported no correlation between Ki67 expression and cell differentiation grade, which is inconsistent with present and above-mentioned studies (33). Lin et al. reported a significant correlation between Ki67 expression and age and distant metastasis in CRC tissue specimens, while the expression had no significant correlations with gender, tumor site, size, and lymph node involvement as well as differentiation degree. They showed that overexpression of Ki67 in CRC was significantly associated with low survival rate, tumorgenesis, and metastasis of cancer cells, and that this protein could be used as a biomarker in prognosis of CRC patients (31).
Given the role of cell proliferation factors in regulating and control epithelial- mesenchymal transition (EMT) in tumor cells, uncontrolled molecular mechanisms of cell cycles seem to cause different behaviors in cancer cells (42), which in turn can justify the difference between Ki67 biomarker expression and its correlation with clinicopathological features.
The findings of the current study on HER2 expression status revealed that it was expressed in 54% of adenocarcinoma samples and its expression in CRC samples was significantly higher than adenoma and normal tissues. However, other studies conducted in recent years reported overexpression of HER2 in CRC between 0 and 80% (43, 44) and there are many debates on its importance as a prognostic marker (44, 45).
In a retrospective cohort study conducted by Seo et al. on biopsy samples of CRCs, HER2 overexpression was reported in 6% of cases. They also found no correlation between HER2 status and clinicopathological characteristics, except for primary location of tumor. However, they stated that HER2 overexpression was associated with its gene amplification (46). Ingold Heppner et al. reported 1.6% positive HER2 expression rate in CRC samples. Their study showed that HER2 overexpression was correlated with the high stage of tumor and lymph node metastasis and local tumor growth (47). While our study revealed that there was not significant association regarding HER2 positive expression and primary location of tumor and lymph node metastasis, therefore, their results are inconsistent with findings of the present study.
Lim et al., in their study on 141 patients with nonmuscle invasive urothelial bladder cancer (NMIBC), reported that HER2 IHC expression rate was accounted for 8.8%. All of HER2 positive expressions occurred in tissues with high grades of NMIBC. Besides, the high expression of HER2 was accompnied by tomur progression, lymph node, and vascular invasion, but not corrolated with other clinicopathological parameters like tomur size and multifocality (48).
The results of these studies had higher differences with our study in terms of HER2 expression. As incidence rate of HER2 in studies is controversial, this variation can be due to various reasons, such as racial and genetic diversity, type of primary anti-bodies used, heterogeneity in studied population, the sample size, discrepancy in technical approach, diversity in type of scoring system, and even type of samples studied (biopsy or tissue resected).
In another study conducted on HER2 expression in colon adenocarcinoma and its correlation with clinicopathological variables, HER2 positive expression was reported in 59.4% of the samples, and its expression was significantly correlated with cancer stages so that its expression reduced at the higher stages of cancer. Additionally, significant relationship was not found among age, gender, tumor location, and type of tumor with HER2 expression in their study (49).
Park et al., studied the clinical importance of HER2 expression in CRC. They reported that HER2 overexpression was 12.5% and stated that tumors with HER2 overexpression were associated with lymph node metastases and lower survival rate (50).
Based on the obtained evidence and expression status of HER2 in a number of papers, HER2 overexpression and gene amplification were utilized to target therapy in many cancers (51). Treatment with transtuzvmab in breast and gastric cancer is widely used based on HER2 expression status now (51, 52).
Considering different and controversial views on HER2 expression in various studies, it seems that HER2 expression is affected by several factors, and differences in expressions of HER2 in cancer tissues could be considered a reflection of biological behaviors of tumoral cells (53). It seems that these cells show different behaviors, according to various conditions, leading to different expressions of HER2. Therefore, according to overexpression rate of HER2 in CRC (54%), this biomarker can be used as a potential tumor and predictor marker for target therapy in CRC.
Despite the controversial expression rate of HER2 protein in many studies that can be due to IHC technique limitations, differences in histological grade, tumor stages, sample size, and geographical location, the crucial role of genetic differences in HER2 expression cannot be ignored. Nonetheless, significant variation of this proto-oncogene expression in CRCs tissues compared with the adenomatous and normal tissues can be utilized for potential targeting treatment.
One of the strong points of our study was that the evaluation of Ki67 and HER2 IHC expressions as cancer biomarkers and their relationship with clinicopathological parameters of colorectal patients with cancer are novel issues, and there is a little data on that in the southeast of Iran.
In conclusion, according to the results obtained in present study, it seems that Ki67 and HER2 protein expressions could be used as beneficial independent prognostic factors in clinical evaluations of patients in prognosis of the situation and progression of the disease and utilized to targeting treatments. IHC can be used as a primary screening technique to identify patients. Further studies with a larger sample size and considering geographical, racial, and genetic differences are recommended.