1. Background
There are 1.2 million new cases of colorectal cancers being diagnosed yearly all over the world, making about 10% of all newly diagnosed malignancies (1). The mortality rate of colorectal cancers is beyond half million each year. In Iran, the reported annual rate of incident colorectal cancer is 5,000 (2). The most important risk factor for colorectal cancer is age, and the incidence significantly rises in people over 45 to 50 years (3, 4). This paradigm was also evident in Iran (5). Due to the relatively high prevalence of colorectal cancer and the fact that the age of patients with colorectal cancer is decreasing, adapting appropriate treatment methods and evaluating the response to treatment, as well as, regular follow ups after treatment completion have gained special importance in these patients.
During the last 3 decades, the outcomes of patients with colorectal cancers in each stage of the disease have improved significantly that is attributed to the improvements in treatment methods (6). In the past, surgery alone was the main treatment for rectal cancer and despite complete resections, up to half of the patients experienced local recurrence (7). By accumulation of evidences, multimodality therapy consisting of various combinations of chemotherapy and radiotherapy protocols led to demarcated improvement both in local control and survival. Although either neoadjuvant or adjuvant radiation therapy is effective in rectal cancers, the former is accepted as the standard treatment. Neoadjuvant radiotherapy usually with concomitant chemotherapy results in downstaging of the tumor, increasing the probability of a better surgery, and the enhancement of sphincter preservation probability in distal rectal cancers. European studies have shown that neoadjuvant treatment results in an improvement in the local control and overall survival of patients with rectal cancer and these results have had a tremendous effect on the treatment of rectal cancer (8).
Since Iran cancer institute and its radiation oncology department is considered a high volume referral center for the treatment of colorectal cancer and due to the numerous and various patients being referred to this center, multiple treatment protocols have been experienced in the management of colorectal cancer. The aim of this study was to evaluate the oncologic outcomes of neoadjuvant long-course radiochemotherapy in order to improve further protocols.
2. Methods
2.1. Participants and Selection Criteria
We performed a retrospective cohort study of all consecutive patients with biopsy proven locally advanced (cT3-4 or N+) rectal adenocarcinoma located up to 15 cm from anal verge by endorectal ultrasound or magnetic resonance imaging. Subjects had to be treated with neoadjuvant chemoradiation followed by surgery at the cancer institute of Imam Khomeini Hospital Complex, Tehran, Iran between 2008 and 2015. We also included a few number of patients with T2N0, who sought sphincter preservation by neoadjuvant radiotherapy. Patients with distant metastases at presentation, concurrent malignancies, and prior pelvic radiation, short course external radiotherapy, and intraluminal rectal radiotherapy were excluded from the study. The study design was evaluated and confirmed by both institutional review board and ethics committee (ethical confirmation code: IR.TUMS.REC.1394.1207). Patients provided written informed consent at the time of treatment initiation.
2.2. Treatment Protocols
Patients were treated with external three-dimensional radiotherapy (6 - 18 Mega Volt energy beams) with the dose range of 45 to 50.4 Gray and concurrent 5flourouracil-based chemotherapy (mainly single agent capecitabin or bolus 5FU). Surgery, generally, was performed no less than 6 to 8 weeks following the completion of radiotherapy and included low anterior resection or abdominoperineal resection. The decision to preserve sphincter was made by post-radiotherapy status of tumor in preoperative colonoscopic exam. Pre-operative (induction and/or consolidation) and post-operative chemotherapy was according to patient compliance as well as at the discretion of treating physician according to presurgical stage and pathologic response to neoadjuvant treatment. The therapy mainly consisted of oral capecitabin and intravenous oxaliplatin for a total of 4 to 6 cycles.
2.3. Assessment and Follow Up
Standard pathologic tumor staging of the resected specimen was performed after resection. Complete response was defined as the absence of viable adenocarcinoma cells in the surgical specimen (ypT0N0). Intermediate response was defined as an improvement in stage to ypT1-2 and ypN0. Patients with ypT3-4 or positive lymph nodes were classified as poor response. Post-operative follow up consisted of routine history and physical examination with carcinoembryonic anti-gen (CEA) measurements every 3 to 6 months along with colonoscopy and computed tomography scans as indicated.
2.4. Outcomes and Analysis
Overall survival (OS) was determined by the time from the end of radiation treatment to death due to any cause or last follow up for survived individuals. Disease-free survival (DFS) was determined by the time from the end of radiation therapy to recurrence or death due to any cause or last follows up.
Gathered data was analyzed by SPSS (Version 21) software (IBM, Chicago, IL). We used the Kaplan-Meier and cox proportional hazards test to assess disease-free survival and overall survival and their predictors. P values less than 0.05 were considered significant.
3. Results
Between 2008 and 2014, about 400 patients with rectal adenocarcinoma were treated in our center. Of these patients, 270 underwent neoadjuvant radiochemotherapy. Finally, 158 subjects were entered to analysis that were treated with standard long-course external beam radiotherapy with concomitant chemotherapy and referred after extirpative rectal surgery with pathology report. The male to female ratio was 1.36:1 and the median age was 56 years (range: 24 - 83). Stage II to III ratio was 31:127. The characteristics of the patients are depicted in Table 1.
| Clinical Characteristic | Results | |
|---|---|---|
| Gender | Male | 91 (57.6) |
| Female | 67 (42.4) | |
| Location, cm above Av | < or = 5 | 75 (47.5) |
| > 5 to 10 | 60 (38) | |
| > 10 | 23 (14.6) | |
| Clinical T | T2 | 19 (12) |
| T3 | 124 (78.5) | |
| T4 | 15 (9.5) | |
| Clinical N | N0 | 31 (19.6) |
| N1 | 81 (51.3) | |
| N2 | 45 (28.5) | |
| Clinical Stage | II | 31 (19.6) |
| III | 127 (80.4) | |
| CEA | High (over 5 nanograms per milliliter (ng/mL)) | 79 (50) |
| Preoperative Chemotherapy (induction and/or consolidation) | 51 (32.3) | |
| Radiotherapy to Surgery Interval, w | < 8 | 58 (36.7) |
| ≥ 8 | 100 (63.3) | |
Clinical Characteristic of Patientsa
The median interval from the last fraction of radiation therapy (RT) to surgery was 9 weeks (range: 1 - 74, Mean ± SD: 10.9 ± 7.79). The wide gap ex parte was due to the complicated patients, whose conditions necessitated emergent surgery; on the other hand, some patients experienced cardiac or medical complications requiring long recovery time before surgery. Pathologic complete, intermediate, and poor responses were achieved in 48 (30.4%), 37 (23.4%), and 73 (46.2%) patients, respectively. Overall down-staging (surgical stage < primary clinical stage) was observed among 116 (73.4%), while tumor and node down-staging occurred in 100 (63.3%) and 105 (66.5%) subjects, respectively. Only one of the participants was ypT0N1-2, representing about 2% of ypT0 cases. T down-staging occurred in 57.9%, 62.2%, and 100% of patients with clinical T2, 3, and 4 tumors (P = 0.011). However, N down-staging was observed in 80% and 93.2% of clinical N1 and 2 tumors (P = 0.068). The pathologic characteristics of surgery specimens are shown in Table 2 and Figures 1 - 4.
| Tumor Characteristics | Results | |
|---|---|---|
| Resected lymph nodes | Median (range) | 4 (0 - 62) |
| Mean ± SD | 5.2 ± 7.05 | |
| Positive PNI | 17 (11.1) | |
| Positive LVI | 23 (14.6) | |
| Involved Margin | 9 (5.9) | |
| yp T | 0 | 49 (31) |
| 1 | 6 (3.8) | |
| 2 | 36 (22.8) | |
| 3 | 64 (40.5) | |
| 4 | 1 (0.7) | |
| yp N | 0 | 129 (81.7) |
| 1 | 21 (13.1) | |
| 2 | 8 (5.2) | |
| yp Stage | 0 | 49 (31) |
| I | 36 (22.8) | |
| II | 45 (28.5) | |
| III | 28 (17.7) |
Tumor Characteristics in Surgical Specimena
In order to predict response to neoadjuvant treatment, in univariate analysis clinical T stage was associated with better outcomes so as the rate of complete pathologic response was more in T2 tumors (47.4%) compared to more advanced stages. In contrast, age (P = 0.931) and interval from RT to surgery (0.085) was not associated with improved response; although, there was a trend toward better response by increasing radiotherapy to surgery interval (mean intervals for complete, intermediate, and poor responses were 12.0, 12.4, and 9.4 weeks, respectively). Among other studied factors as presented in Table 3, sex, location, clinical stage, clinical N, and preop chemotherapy were not associated with pathologic response with statistical significance.
| Complete | Intermediate | Poor | Significance | ||
|---|---|---|---|---|---|
| Age Group, y | 50 or less | 15 (29.4) | 13 (25.5) | 23 (45.1) | 0.972 |
| 51 - 65 | 20 (31.7) | 15 (23.8) | 28 (44.4) | ||
| Over 65 | 13 (29.5) | 9 (20.5) | 22 (50) | ||
| Sex | Male | 27 (29.7) | 24 (26.4) | 40 (44) | 0.585 |
| Female | 21 (31.3) | 13 (19.4) | 33 (49.3) | ||
| Location, cm above Av | < or = 5 | 24 (32) | 20 (26.7) | 31 (41.3) | 0.373 |
| > 5 to 10 | 20 (33.3) | 10 (16.7) | 30 (50) | ||
| > 10 | 4 (17.4) | 7 (30.4) | 12 (52.2) | ||
| Clinical T | T2 | 9 (47.4) | 7 (36.8) | 3 (15.8) | 0.036 |
| T3 | 37 (29.8) | 25 (20.2) | 62 (50) | ||
| T4 | 2 (13.3) | 5 (33.3) | 8 (53.3) | ||
| Clinical N | N0 | 14 (45.2) | 8 (25.8) | 9 (29) | 0.194 |
| N1 | 23 (28.4) | 17 (21) | 41 (50.6) | ||
| N2 | 10 (22.2) | 12 (26.7) | 23 (51.1) | ||
| Stage | 2 | 14 (45.2) | 8 (25.8) | 9 (29) | 0.07 |
| 3 | 34 (26.8) | 29 (22.8) | 64 (50.4) | ||
| Preoperative chemotherapy | Yes | 16 (31.4) | 14 (27.5) | 21 (41.2) | 0.62 |
| No | 32 (29.9) | 23 (21.5) | 52 (48.6) | ||
| Radiotherapy to Surgery Interval, w | < 8 | 11 (19) | 14 (24.1) | 33 (56.9) | 0.045 |
| ≥ 8 | 37 (37) | 23 (23) | 40 (40) |
Tumor Response Based on Clinical Factors
The median follow up time was 28 (range: 1 - 102) months in our cohort study. The rate of local recurrence was 8.3% among all subjects, while this rate was 2.1%, 5.4%, and 13.9% among subjects with complete, intermediate, and poor response, respectively (P = 0.055). The overall rate of distal recurrence was 14.6% in our study. This rate was 6.2%, 16.2%, and 19.4% among subjects with complete, intermediate, and poor response, respectively (P = 0.128). Among recurred cases, 3 patients suffered from both local and distal; 20 only distant and 10 only local recurrences.
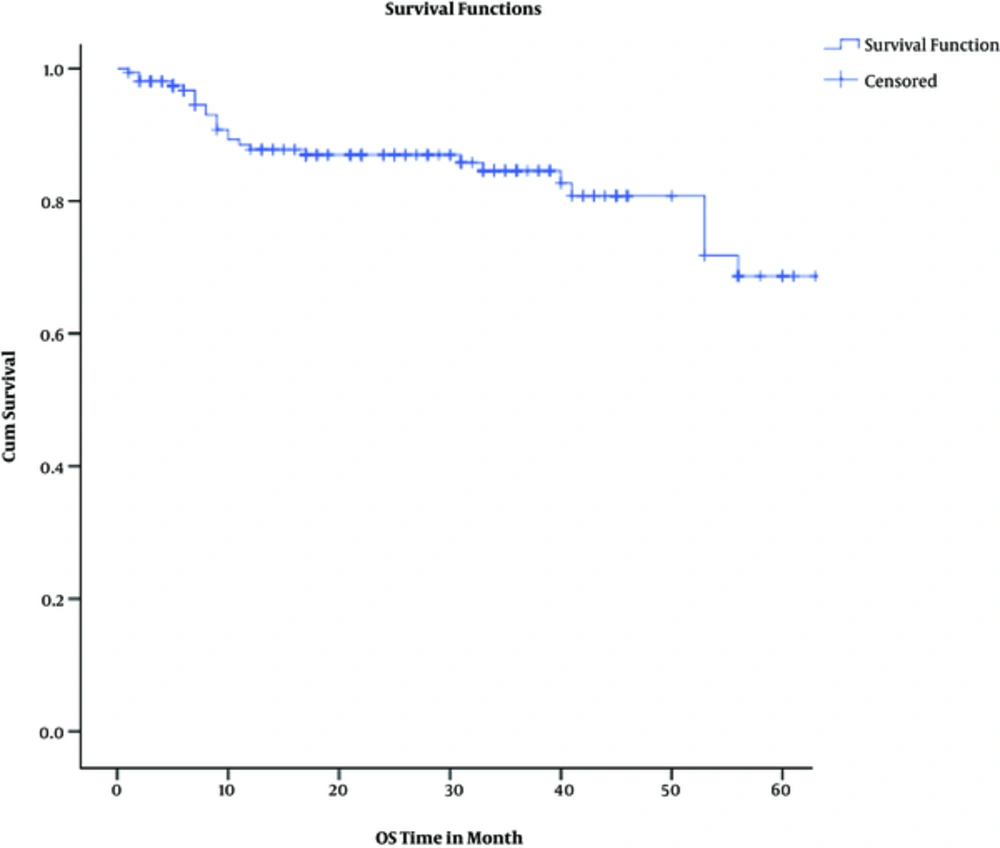
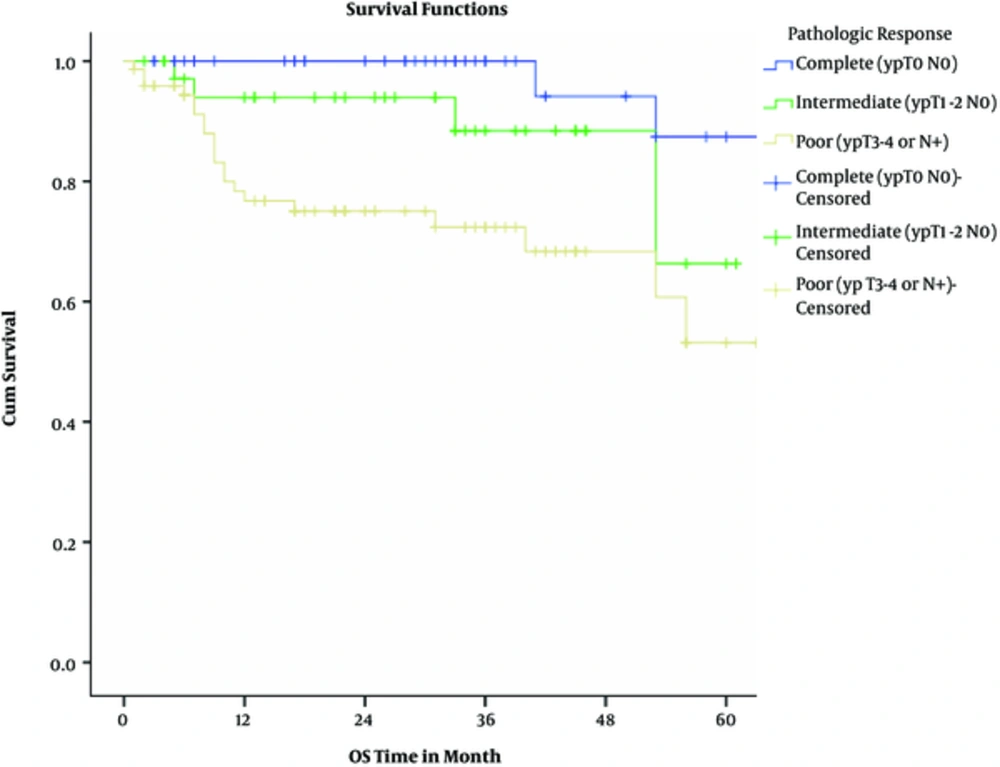
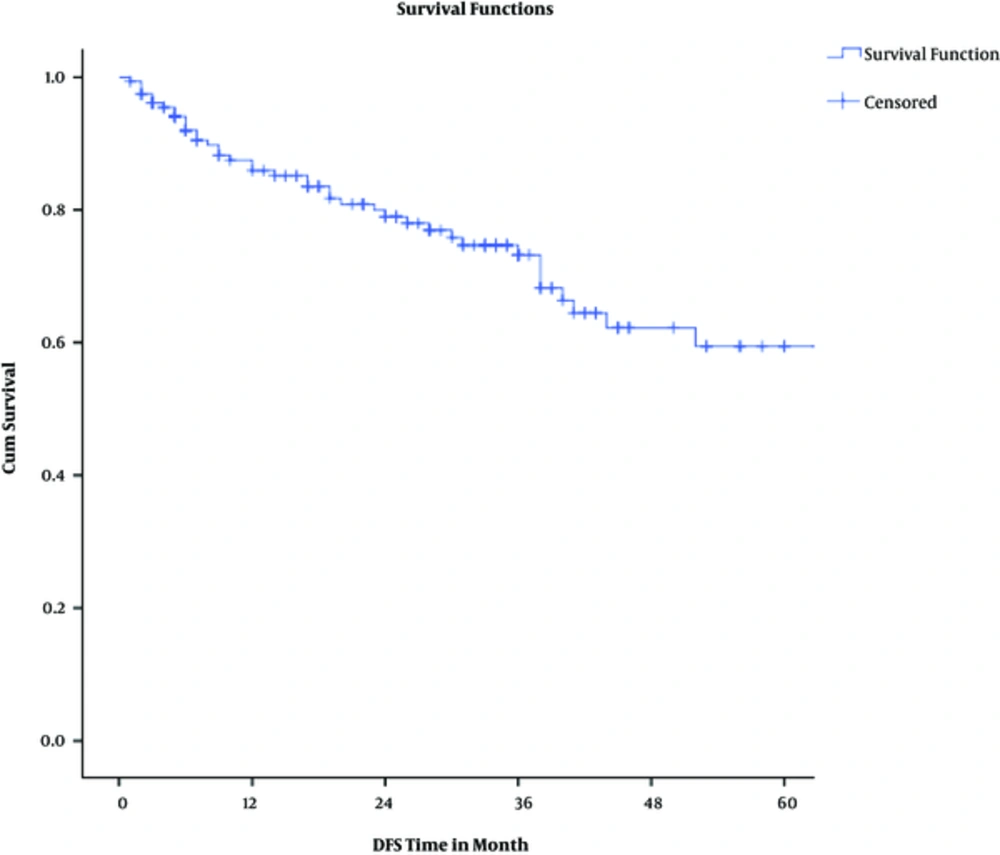
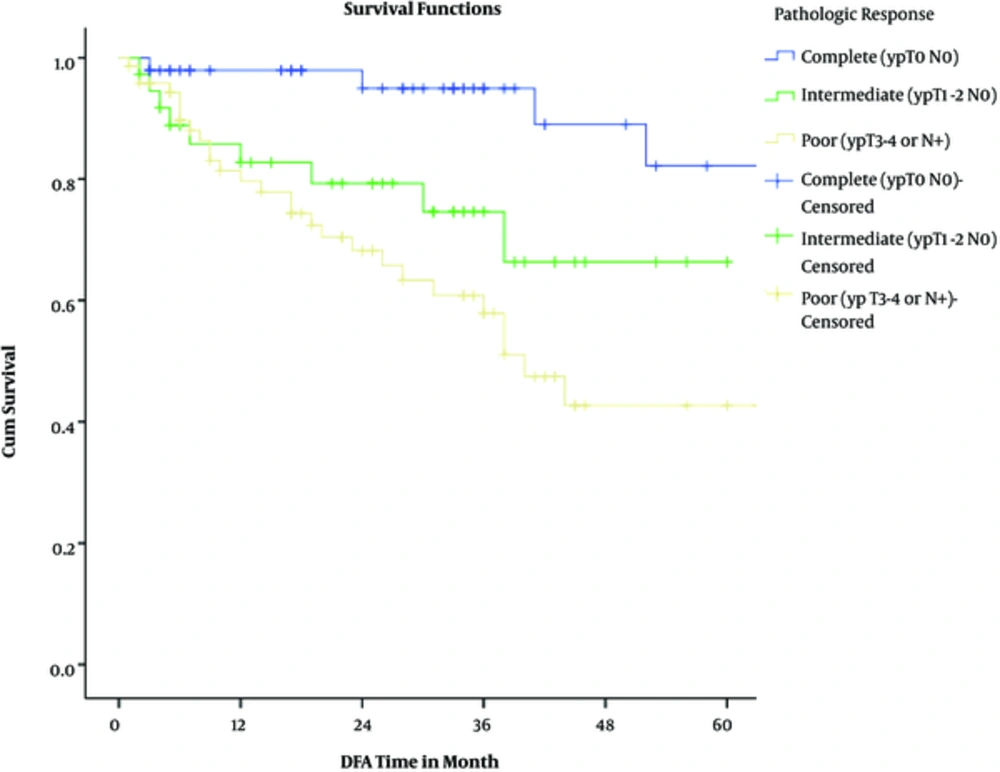
Actuarial 2-year overall and disease-free survival rates were 87% and 80%, respectively. Considering pathologic response, 2-year overall survival rates were 100%, 94%, and 86% among complete, intermediate, and poor responders (P = 0.00043). Two-year disease-free survival rates were 98%, 79%, and 68% among complete, intermediate, and poor responders (P = 0.0013).
The results of univariate cox regression analysis for 2-year overall survival are shown in Table 4; Multivariate cox proportional hazards test showed that only pathologic response (P = 0.035) and distant metastasis (P < 0.0001) were 3 independent predictors of overall survival. Again, pathologic response (P = 0.009) was the only independent predictor of disease-free survival in multivariate analysis adjusted model.
| OS, % | Significance | ||
|---|---|---|---|
| Age group, y | 50 or less | 91 | 0.679 |
| 51 - 65 | 83 | ||
| Over 65 | 90 | ||
| Sex | Male | 84 | 0.124 |
| Female | 91 | ||
| Distance from anal verge, cm | 5 or less | 87 | 0.943 |
| 6 - 10 | 89 | ||
| Above 10 | 86 | ||
| Clinical T | T2 | 100 | 0.184 |
| T3 | 87 | ||
| T4 | 79 | ||
| Clinical N | N0 | 100 | 0.209 |
| N1 | 86 | ||
| N2 | 82 | ||
| Stage | 2 | 100 | 0.095 |
| 3 | 84 | ||
| Preop Chemo | Yes | 88 | 0.808 |
| No | 87 | ||
| Radiotherapy to Surgery Interval, w | < 8 | 84 | 0.413 |
| ≥ 8 | 89 | ||
| T Downstaging | Yes | 85 | 0.299 |
| No | 90 | ||
| N Downstaging | Yes | 93 | 0.500 |
| No | 86 | ||
| Surgical Margin | Involved | 65 | 0.001 |
| Free | 90 | ||
| Local Recurrence | Yes | 92 | 0.469 |
| No | 88 | ||
| Distant Recurrence | Yes | 64 | < 0.00001 |
| No | 92 |
Predictors of 2-Year Overall Survival
4. Discussion
The results of this retrospective study contain valuable information about the various aspects of rectal cancer, especially on treatment protocols. Near three-fourths of all subjects enjoyed down-staging by neoadjuvant chemoradiotherapy. In addition, a third of the patients experienced pathologic complete response (pCR), while near half showed poor response to neoadjuvant treatment. Initial clinical T stage was a strong predictor for pCR. During follow up period, the rate of local and distant failures were roughly around 8% and 15%, respectively; both were significantly higher among poor responders. Two-year overall and disease-free survival rates were 87% and 80%, respectively. For OS and DFS, the pathologic response was a significant independent predictor.
The median age of patients with rectal cancer was 56 years, in concordance with other epidemiologic studies in Iran (9-11); however, it is less than the median age in western countries that is 64 years. According to Ansari et al. although the incidence of rectal cancer among people aged 45 to 54 years in the United States is 2.5 times more frequent than Iran, this gap reaches 9 times in persons above 65 years (12). The change of lifestyle among the youth and younger age pyramid in Iran are among explanations (13, 14).
The rate of pathologic complete response (pCR) in our study (30%) is higher than the most of large studies evaluating neoadjuvant treatment in rectal cancer. In Germans' study (CAO/ARO/AIO-94) published in 2004, which is called a milestone research, the reported pCR was only 8% (8). The PAN-EX study, a pooled analysis of 2 studies EXPERT and EXPERT-C, reported 19% pCR. The rate of pCR in ACCORD12/0405-Prodige 2 trial, published in 2010, was 14% in the standard arm and 19% in the group receiving oxaliplatin concurrent with radiation (15). In contrast, some studies report the high rates of pCR. For instance, the rate of pCR was 33% in studies conducted by Perez et al. and Marechal et al. evaluating 39 and 57 patients, respectively (16, 17). One of the likely reasons of this difference is the interval between the completion of radiotherapy and surgery. This interval in the majority of older studies was between 6 to 7 weeks, while in our study, about 64% underwent surgery 8 weeks or later after the completion of radiotherapy (RT). There are numerous studies indicating that increasing interval between the completion of RT and surgery leads to an increase in pCR in rectal cancer (18). The mean number of resected lymph nodes in our study was 5.2, while this number is usually more than 10 in other studies. For example, the mean number of resected lymph nodes in the German CAO/ARO/AIO-04 and ACCORD12/0405-Prodige2 studies were 15 and 12, respectively (15, 19). Therefore, there is a possibility that the reason why some of our patients were ypN0 was the inadequate resection of lymph nodes during surgery or the inadequate pathologic review of the specimen. The rate of ypN0 in the 2 previously mentioned studies was about 71% compared to 81% in our study. The rates of primary cN+ in those studies and in the present study were 72% and 80%, respectively. Perhaps one would argue that this less number of resected lymph nodes is one of the possible reasons for high pCR rates. Nonetheless, we should consider the fact that the most important determinant of pCR rate is the amount of ypT0, as there are few instances, in which ypT0 is accompanied by ypN+. For example, in the CAO/ARO/AIO-04 study, among the 83 patients achieving ypT0 in the group receiving Fluorouracil (without oxaliplatin), only 2 were ypN+ (2.4%) (11). Similarly, among 49 patients with ypT0 in our study, only 1 had ypN+ (2%). Therefore, the overall impact of this factor on the rate of pCR will not be much.
The relationship between pCR and survival have been demonstrated in various studies, prompting many investigators to suggest the “watch and wait” strategy in patients achieving clinical CR, of course with close monitoring (20-23). The association of pathologic response and survival has been significant in our study as well. Nevertheless, despite the higher rates of pCR in this study, overall and disease-free survival is as good as other large studies or slightly worse. However, these numbers are significantly higher compared to the median survival of patients with rectal cancer in Iran. In studies conducted by Moradi et al. and Akhoond et al. the 2-year survival was 68% to 74% and the median survival was 3.5 to 3.9 years, respectively (24, 25).
About 6% of our patients had positive surgical margin that is in line with the literature. The rate of positive margins was 3% to 4% in German CAO/ARO/AIO-04 study, 6% to 7% in Sauer 2004 study, and 7.7% and 12.7% in 2 arms of ACCORD12/0405-Prodige2 study (circumferential margin) (8, 15, 19).
In our study, although pre-operative (induction and/or consolidation) chemotherapy led to higher rate of complete response, it did not lead to improved survival. Induction chemotherapy allows administering higher doses and longer exposure time to cytotoxic agents and in theory could lead to more down-staging of tumor and lymph nodes and faster eradication of micrometastatic disease (26, 27). To date, many phase II trials have investigated this matter. The phase II study of Marechal et al. comparing neoadjuvant chemoradiation with or without 2 courses of induction chemotherapy with FOLFOX was prematurely closed for futility (16). In the GCR-3 study (conducted by Fernandez-Martos) that compared neoadjuvant chemoradiation with the addition of 4 courses of adjuvant or neoadjuvant chemotherapy with CapeOx regimen, no differences were observed between the 2 groups regarding the rate of pCR or complete resection, distant metastases, 5-year DFS, or 5-year overall survival. However, patients tolerated the induction chemotherapy better than the adjuvant one (28). In our study, 37 patients were treated with induction chemotherapy. Among them, 10 achieved pCR (27%), not significantly different from the group that did not receive it (31.4%). Also, the survival of patients did not have any association with receiving induction chemotherapy and the number of cycles. According to the literature to date, neoadjuvant chemoradiation is still the standard treatment. Although, based on the results of Fernandez-Martos study, the NCCN guidelines consider induction chemotherapy as a treatment option (29). Another factor evaluated in our study was the administration of chemotherapy during the interval between radiation treatment and surgery. Overall, 20 patients received preoperative consolidation chemotherapy in our study, 9 of them achieving pCR (45%). This percentage is higher in comparison with the percentage of pCR in the group not receiving this therapy (28.3%); however, this difference did not reach statistical significance due to small sample size. Some studies showed that adding pre-operative chemotherapy results in modest increase in pCR rate without increasing complications (30). The main advantage of earlier administration of chemotherapy may be better tolerance of patients. Delivering chemotherapy during the interval between chemoradiotherapy and surgery decreases the overall treatment time. Also, not evaluated in our study, the administration of pre-operative consoliadative chemotherapy is more pleasant for many patients treated in Iran, since the long period between radiation to surgery leads patients to think that they have been abandoned. This issue may be the result of cultural traits or the conditions of clinics in Iran.
Factors associated with overall survival in our study included the rate of pathologic response of tumor to neoadjuvant treatment and distant metastases.
Our study had a number of limitations as well. The major limitation was very short median follow up time compared to the expected long prognosis of rectal cancer. As mentioned previously, the retrospective nature of our study makes it hard to interpret the results. Also, we did not have access to some peri-treatment information of patients. For instance, the information about treatment morbidities or the status of circumferential radial margin were not accessible in most cases and, therefore, were not reported.
In summary our study showed that pathologic response to neoadjuvant therapy could be taken into account as a surrogate for long-term oncologic outcomes such as overall survival. Although the meaningful effect of longer interval between the completion of radiation and surgery on pathologic response did not equal to better overall survival, it is recommended to keep the 8 week interval. There are no meticulous data on the role of induction or consolidation chemotherapy, but the observed pathologic responses merits further investigations. Our promising results may point out the necessity of treating patients with locally advanced rectal cancer in high volume comprehensive centers.
This study was completely conducted in radiation oncology ward, Iran cancer institute, Imam Khomeini hospital complex, Tehran University of Medical Sciences