1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has become a major challenge for public health and clinical medicine. COVID-19 is caused by severe acute respiratory coronavirus-2 (SARS-COV-2). The disease typically presents with pulmonary symptoms such as cough, dyspnea, chest pain, and fever (1). Gastrointestinal symptoms such as diarrhea, abdominal pain, nausea, and vomiting are also increasingly seen in patients with COVID-19 (2). Acute pancreatitis is seen in other viral infections such as coxsackievirus, herpes viruses, and hepatitis viruses. Pancreatic involvement has been reported in some cases in COVID-19, although its pathogenesis is unknown (2).
Here, we reported a case of acute pancreatitis after COVID-19 in a child with acute lymphoblastic leukemia (ALL).
2. Case Presentation
A 4-year-old boy, a known case of standard risk ALL (3), was admitted to the emergency department with anorexia, severe epigastric pain, nausea, and vomiting since 3 days before. He was in the consolidation phase at the time of admission. Vomiting was bilious and non-bloody. He did not have fever, jaundice, cough, diarrhea, or shortness of breath. On physical examination, he was afebrile and ill appearance. He had moderate dehydration. Abdomen examination revealed epigastric tenderness and mild distention. His oxygen saturation in room air was 96%. The present case was undergoing chemotherapy based on ALL IC-BFM 2002 protocol (3). He was in complete remission at the end of the induction phase.
The complete blood count was as follows: White blood cell count, 2 × 103/mm3 with 32% lymphocyte; MCV, 88 fL; hemoglobin, 10.8 g/dL; and platelet count, 310 × 103/mm3. Serum Amylase was 285 U/L (normal range, 30 - 100 U/L) and serum lipase was 264 U/L (normal range, 13 - 60 U/L). Potassium: 2.2 mEq/L, calcium: 7 mg/dL, phosphorus: 2 mg/dL, albumin: 3.3 g/dL, prothrombin time (PT): 16.3 sec, partial thromboplastin time (PTT): 44 sec, INR: 1.7. Erythrocyte sedimentation rate (ESR) was 64 mm/hr. Other laboratory tests including urea, creatinine, blood glucose, alanine transferase (ALT), spartate aminotransferase (AST), alkaline phosphatase (ALP), sodium, total and direct bilirubin were in the normal range.
Nasopharyngeal swab was positive for COVID-19 reverse transcriptase polymerase chain reaction (RT-PCR). Other family members were negative for COVID-19.
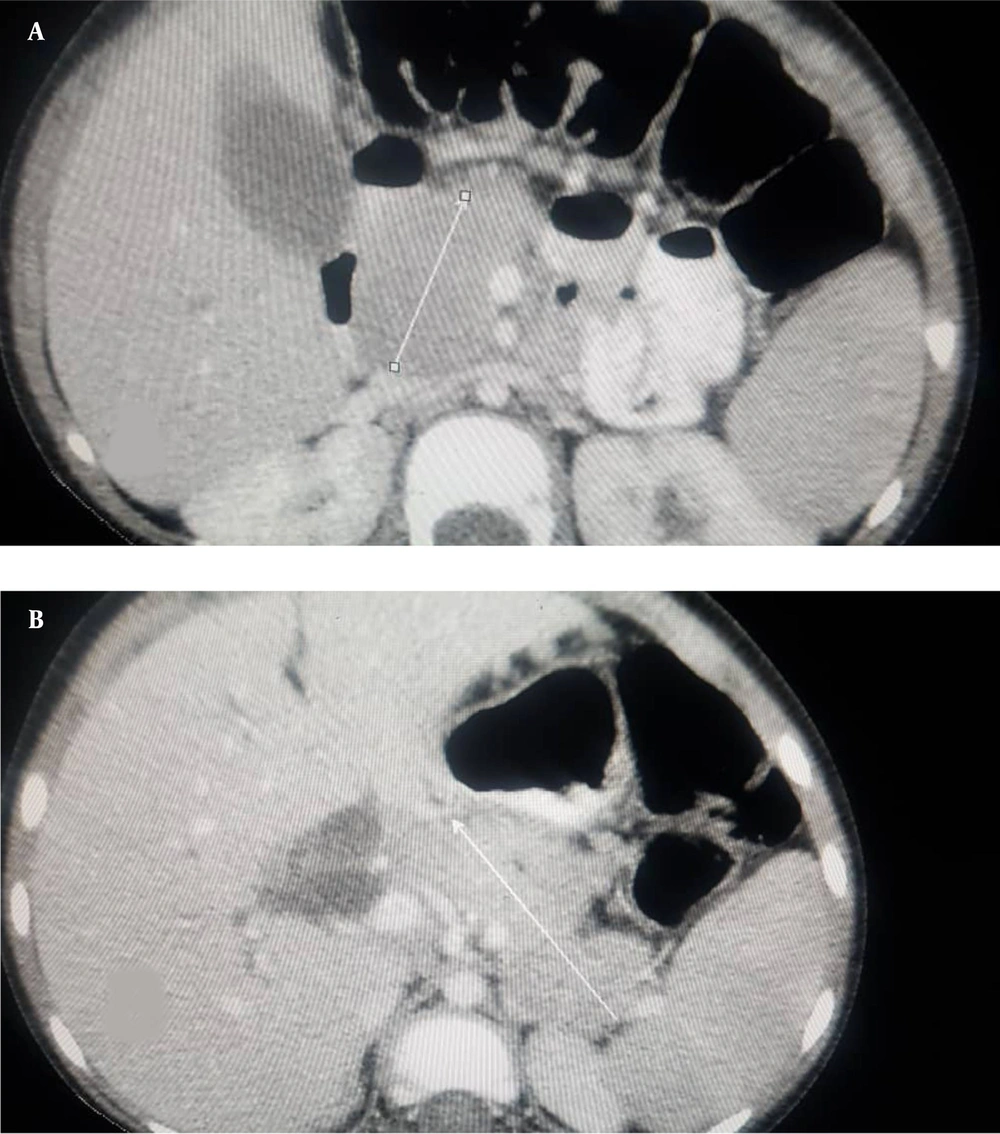
Abdominal ultrasound showed moderate free fluid and diffuse enlargement of the pancreas. Spiral computed tomography scan of the lung showed presence of moderate fluid in the left pleural cavity without evidence of ground glass opacity. Contrast-enhanced abdominal CT scan showed features of necrotizing pancreatitis. It also revealed edematous and enlarged pancreas, non-enhancing areas in the head, neck, body and tail of the pancreas and moderate free fluid in the abdominal cavity and pelvis (Figure 1). Based on the history, physical examination, laboratory findings, and imaging, the diagnosis of acute pancreatitis secondary to COVID-19 was confirmed in this patient. The patient was admitted to an isolated room.
Supportive care including intravenous fluid therapy, bowel rest, gastric drainage via nasogastric tube, electrolyte supplementation, antibiotics, analgesic, and pantoprazole were initiated.
After three days, the abdominal pain improved and the biliary discharge stopped. One week after admission, the patient was discharged without complications and in good general condition.
At the time of writing this report, the patient is in the maintenance phase of treatment.
3. Discussion
Acute pancreatitis in childhood ALL is usually caused by medications such as L-asparginase, peg-asparginase, and steroids. About 80 - 90% of cases are mild and in a small percentage may be hemorrhagic or necrotizing (4). The incidence of drug-induced pancreatitis in childhood ALL is 6.7 - 18% (5). Raja et al. (5) reported asparginase-induced pancreatitis occurs after the first few doses (median of 5 doses). It has also been observed that on average, pancreatitis occurs 4 to 11 days after the last dose of asparaginase (6, 7).
In a retrospective study in New York, 1.8 % of people under 18 with COVID-19 had pancreatitis, compared with 0.14% in the non-COVID-19 population (8).
In a study by Samies et al. (9) three children aged 11, 15 and 16 reported with pancreatitis after COVID-19 infection. In two studies in the USA and Spain, acute pancreatitis was reported in 0.27% and 0.07% of hospitalized adults due to COVID-19, respectively (10, 11).
None of the cases of acute pancreatitis reported in children following COVID-19 were present in children with acute leukemia. In the present case, we did not find any other risk factor for acute pancreatitis except COVID-19. Our patient received the last dose of asparaginase one month ago. Therefore, asparinginase-induced pancreatitis is less likely in our patient.
Pancreatitis should be considered in any patient with acute lymphoblastic leukemia presenting with abdominal pain. According to COVID-19 epidemics, if acute drug-induced pancreatitis is not present in these patients, viral causes, especially COVID-19, should be considered. Comprehensive care and immediate intensive treatments can improve the patient's prognosis.