1. Background
Since the beginning of the COVID-19 pandemic, patients with cancer have faced several challenges (1). Up to now, we know that patients with cancer are at a high risk of mortality from COVID-19 infection (2, 3). Consequently, they are prioritized for vaccination since it is the most effective way of preventing the disease (4, 5). However, the immune impairments which result from cancer itself or the therapeutics for cancers can affect the vaccine effectiveness (6). To date, there are limited studies about the immunogenicity, safety, and efficacy of inactivated vaccines in preventing COVID-19 infection, hospitalization, and mortality in patients with cancer. A study by Ariamanesh et al. showed that the seroconversion rate in cancer patients receiving the Sinopharm BBIBP-CorV vaccine is 86.9% (7). However, this study did not report any breakthrough infections. Another study by Joudi et al. also found that the seroconversion rate in patients with breast cancer receiving the Sinopharm BBIBP-CorV vaccine is more than 80%, and the breakthrough infection rate is 0.7% (8).
2. Objectives
The aim of this study was to report the patients’ demographics, clinical characteristics, and outcomes of all the patients with any type of cancer at a tertiary referral cancer center who had a positive PCR test after they were fully vaccinated by the Sinopharm BBIBP-CorV COVID-19 vaccine.
3. Methods
3.1. Data Acquisition
Omid cancer hospital is a tertiary referral cancer center affiliated with the Isfahan University of Medical Sciences, also known as Medical University of Isfahan (MUI) in Isfahan, Iran. The data of patients with cancer who reside in Isfahan Province was extracted from the hospital information system (HIS).
The data of patients with a positive SARS-CoV-2 PCR from 21 February 2020 until 16 August 2021 was also gathered by Isfahan COVID-19 Registry (I-CORE) (9). Vaccination status data was also collected by I-CORE.
The data of these two datasets were linked together using the national identification number. We identified breakthrough infections in fully vaccinated patients, defined as a positive test > 14 days after the second vaccine dose (10).
After finding the patients with breakthrough infections, clinical characteristics, comorbidities, signs, and symptoms were extracted from the electronic and paper health records.
3.2. Statistical Analysis
Preprocessing of data was done in Python using Pandas and NumPy libraries. Afterward, data were entered into Statistical Package for the Social Sciences (SPSS) Statistics software version 26. Median with Interquartile range (IQR) and frequency were used to report categorical and continuous variables.
3.3. Ethics Approval
This study was approved by the Research Ethics Committees of Vice-Chancellor in Research, Medical University of Isfahan (Approval ID: IR.MUI.MED.REC.1400.483).
4. Results
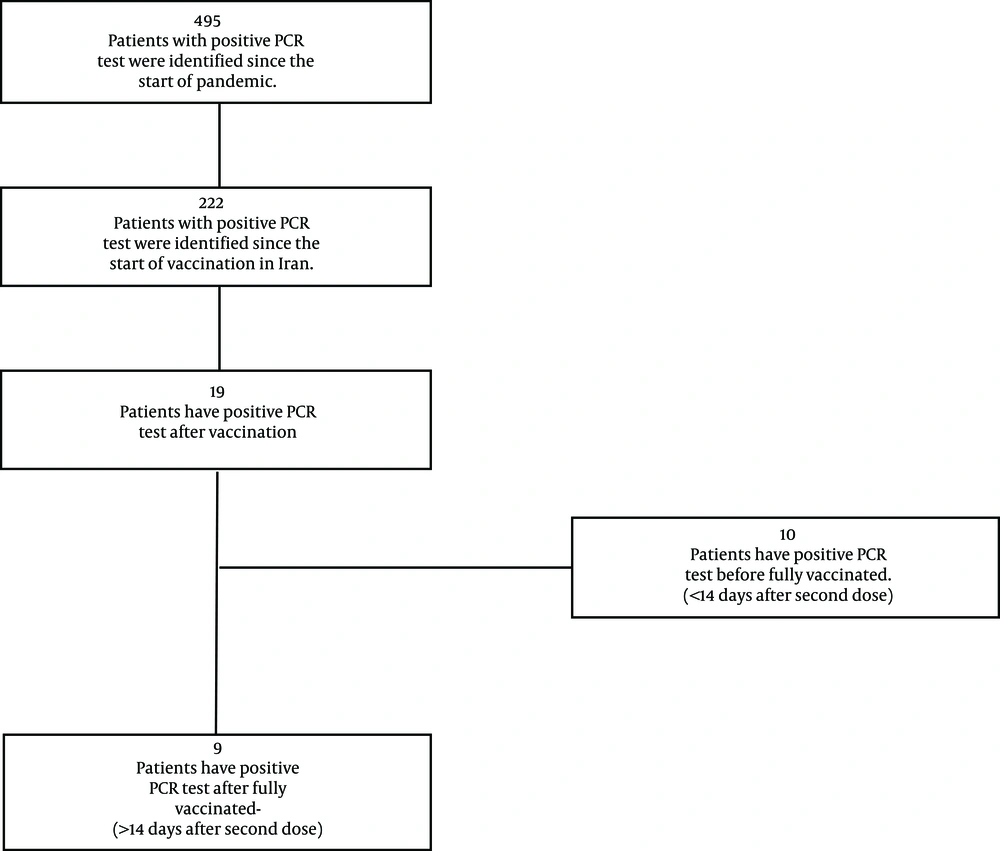
A total of 495 Patients with cancer have had a positive PCR test since 21 February 2020, the day that the first patient with cancer was diagnosed with COVID-19; 222 of them were after the start of COVID-19 vaccination in Iran. Of these, 9 (4.1%) breakthrough infections have occurred (Figure 1). Among them, 6 (67%) were male with a median age of 64, and 6 (67%) had at least one comorbidity besides cancer. Six (67%) have hematological malignancies (Table 1).
| Patient Characteristics | No. (%) |
|---|---|
| Gender | |
| Male | 6 (66.7) |
| Female | 3 (33.3) |
| Age (median, IQR) | 64 (58 - 74) |
| Type of cancer | |
| Hematological | 6 (66.7) |
| Solid tumor | 3 (33.3) |
| Hematological cancers | |
| Multiple myeloma | 2 (22.2) |
| Acute myeloblastic leukemia | 1 (11.1) |
| Mantle cell lymphoma | 1 (11.1) |
| Hodgkin lymphoma | 1 (11.1) |
| Non-hodgkin lymphoma | 1 (11.1) |
| Solid tumors | |
| Breast | 1 (11.1) |
| Thyroid | 1 (11.1) |
| Connective Tissue | 1 (11.1) |
| Comorbidities | |
| Hypertension | 3 (33.3) |
| Other cardiovascular diseases | 2 (22.2) |
| Diabetes | 2 (22.2) |
| Chronic kidney disease | 2 (22.2) |
| Dialysis | 1 (11.1) |
| Cancer Treatments | |
| Recent chemotherapy (within four weeks of admission) | 3 (33.3) |
| Days from second vaccine dose to infection (median, IQR) | 60 (56 - 69) |
| Have any symptoms | |
| Yes | 8 (88.9) |
| No | 1 (11.1) |
| Symptoms | |
| Fever | 6 (66.7) |
| Cough | 6 (66.7) |
| Dyspnea | 5 (55.5) |
| Malaise | 7 (77.8) |
| Myalgia | 6 (66.7) |
| Headache | 1 (11.1) |
| Diarrhea/vomiting/nausea | 3 (33.3) |
| Anosmia | 3 (33.3) |
| Dysgeusia | 2 (22.2) |
| Chest Pain | 3 (33.3) |
| Outcomes | |
| Outpatient only | 3 (33.3) |
| Hospitalization | 6 (66.6) |
| Death | 3 (33.3v |
All the patients received the Sinopharm BBIBP-CorV vaccine. Eight (88.9%) patients were symptomatic, and 6 (67%) were hospitalized. The median time from the final vaccine dose to infection was 60 days. At last, 3 (33%) of the patients died. Two of them had recent chemotherapy and hematological malignancy. The chemotherapy regimen of one of these patients included rituximab, and the other one received bortezomib and lenalidomide. The information about these patients is available in Table 2.
| Case | Age | Cancer Type | Time from Final Vaccine to Diagnosis (d) | Recent Chemotherapy | Asymptomatic Infection | Hospitalization | Deceased |
|---|---|---|---|---|---|---|---|
| 1 | 56 | Acute myeloblastic leukemia | 43 | No | Yes | No | No |
| 2 | 64 | Multiple myeloma | 69 | Yes | No | Yes | Yes |
| 3 | 73 | Mantle cell lymphoma | 61 | Yes | No | Yes | Yes |
| 4 | 74 | Malignant neoplasm of breast | 44 | No | No | No | No |
| 5 | 32 | Hodgkin lymphoma | 83 | Yes | No | Yes | No |
| 6 | 58 | Multiple myeloma | 60 | No | No | Yes | No |
| 7 | 75 | Non-hodgkin lymphoma | 56 | No | No | Yes | No |
| 8 | 78 | Malignant neoplasm of connective and soft tissue | 83 | No | No | No | No |
| 9 | 64 | Malignant neoplasm of thyroid | 60 | No | No | Yes | Yes |
5. Discussion
Findings showed that a small portion (4.1%) of patients with cancer in our center were infected with COVID-19 when fully vaccinated. As previous studies showed that the seroconversion rate of the Sinopharm BBIBP-CorV vaccine is more than 80% (7, 8), it seems that the Sinopharm vaccine is an effective method for preventing COVID-19. Other studies also reported the limited number of breakthrough infections in the general population and showed that breakthrough infection is correlated with neutralizing antibody titers (11, 12). There is only a limited number of studies describing breakthrough infections in patients with cancer. A study by Schmidt et al. showed that cancer patients with hematological malignancies are at more risk for breakthrough infections (13). Another study by Pagano et al. described breakthrough infections in patients with hematological malignancies also showed that breakthrough infections are more prevalent in patients with lymphoproliferative malignancies (14). Our findings also show that breakthrough infections are more prevalent in patients with lymphoproliferative malignancies and mortality is more prevalent in cancer patients under active chemotherapy.
Multiple myeloma and mantle cell lymphoma are lymphoid disorders that affect the B cells. As the Sinopharm BIPP vaccine mechanism of action relies on producing neutralizing antibodies (15), it seems that these patients are at increased risk of reinfection.
One of the patients who died received rituximab. A study by Bonelli et al. also showed impaired humoral response after COVID-19 vaccination in patients receiving rituximab (16). It is not surprising that Anti-CD20 antibodies, which deplete the B cells, predispose patients to infections.
5.1. Limitations
This study had several limitations. First, this study was conducted during a period when cancer patients did not receive booster doses. As the booster doses could affect these results, it is highly suggested that other studies describe the breakthrough infections after the booster doses. Moreover, in this study, we did not evaluate the presence of SARS-CoV2 antibodies. Finally, it is worth mentioning that we used a registry database for obtaining the PCR results, and this registry did not record the details of COVID-19 diagnostic kits. As the sensitivity of different kits may differ, it could affect the PCR tests.
5.2. Conclusions
Our study also showed that, although vaccination reduces the chance of getting infected, more than half of cancer patients with breakthrough infection were hospitalized, and a third finally died. This might be because of the emergence of new virus variants or the difference in the effectiveness of vaccines in cancer patients for preventing hospitalization or mortality.
The authors suggest that it is necessary to study the effectiveness of each COVID-19 vaccine for cancer patients to find the best vaccine for these patients. Moreover, Because of the waning immunity of vaccines especially inactivated vaccines, patients with cancer received booster doses to prevent COVID-19 infection (17). As a result, conducting studies comparing the effectiveness of booster vaccines especially those with hematological malignancies or receiving chemotherapies, is highly suggested.