1. Background
Acute radiodermatitis (ARD) affects up to 100% of patients with breast cancer (1, 2). Eighty-five percent of patients develop moderate-to-severe dermatitis. The most reported symptoms are skin dryness, warmth, burning sensations, erythema, desquamation, ulceration, and even necrosis (3, 4). Dermatological changes occur within 1 - 4 weeks following radiotherapy initiation. They are due to DNA damage and inflammatory cytokine release that cause capillary dilation, localized swelling, and leukocyte infiltration (1, 5, 6). They persist for the duration of radiotherapy and may require up to 1 month to heal after treatment completion (7). These side effects have an impact on patients’ quality of life due to pain. It might require suspension of the radiation schedule. Moreover, recent data suggested that ARD was an independent risk factor for late skin toxicities (4). Therefore, the management of ARD is a major concern in patients with breast cancer receiving radiotherapy. Despite the lack of consensus, some treatments have shown efficacy for prophylaxis and management of ARD in breast cancer. They include washing the irradiated skin with soap and water during radiotherapy, systematic treatment with amifostine, topical use of corticosteroids, and some other topical agents (aloe vera, eucerin, trolamine, etc. applied at the onset of ARD or the beginning of radiotherapy (3, 8). Most of them are not available in Middle-East countries.
Phenytoin was introduced in 1937 as an antiseizure medication. Its wound-healing properties were first reported by Sahpiro in 1958 (9). Although the mechanism of action for this feature remains a debated issue, topical phenytoin is widely used for its healing properties in traditional middle east medicine. It is believed to induce fibroblast proliferation, extracellular matrix proteins expression, and growth factors activity enhancement, leading to collagen deposition in the wound (10).
2. Objectives
It is the first time a study has aimed at assessing the efficacy of phenytoin topical ointment on the prophylaxis and treatment of ARD in patients with breast cancer whose undergoing radiotherapy.
3. Methods
The present prospective randomized double-blind placebo-controlled clinical trial has been conducted at the Babol University of Medical Sciences from 2015 to 2018. The protocol was approved by the local Ethics Committee, and was conducted in compliance with the Helsinki Declaration. It was registered in the Iranian Registry of Clinical Trials (www.irct.ir, ID No.: IRCT2012070710205N1). The patient’s informed consent was systematically obtained before treatment initiation.
3.1. Patient Selection
Included patients were 18 - 75-year-old non-metastatic female patients with breast cancer. They were undergone adjuvant external beam irradiation following breast surgery. With a confidence level of 95% and a power of 80%, assuming 95% of patients would develop no severe dermatitis (RTOG ≥ 3) in the treatment group and 70% in the placebo group, the number of 32 samples in each group was estimated, and with a 10% probability of sample drop, 35 samples entered in each group. The volunteered patients were randomly assigned to two groups. Patients received either phenytoin 1% cream (experimental group) or petrolatum cream (placebo group) in blinded tubes which had simply been given a unique confidential code to each tube by a third party (pharmacist). All patients had completed adjuvant postoperative chemotherapy cycles before radiotherapy initiation. Patients with bilateral breast cancer, concomitant chemotherapy, or allergy to phenytoin (or placebo) were excluded.
3.2. Study Design
3.2.1. Topical Creams Administration
Twice a day, patients were asked to apply their topical cream (prepared and blinded by a pharmacist) on irradiated skin from the beginning to completion of radiotherapy. They had to clean the radiation area just before each session of radiation using tap water and neutral soap (Dove (R)).
3.2.2. Radiotherapy
Patients were treated with tangential 6 MV photon beam radiotherapy to the chest wall and supraclavicular area with a linear accelerator (Siemens). Patients were positioned supine with arms up and extended elbows. The cranial and caudal portals were 2 cm from the parenchyma, and the medial field border was in the midline of the patient. The wedges were used to optimize the dose distribution, no bolus was allowed. Radiotherapy was delivered in 2 Gy per fraction, for 5 days a week; the total dose was 50 Gy to whole breast and 10 Gy boost to the tumor bed with 2 cm margins.
3.3. Assessment of Acute Radiation Dermatitis and Statistical Analysis
Acute radiodermatitis was evaluated weekly according to the Radiation Therapy Oncology Group (RTOG) staging criteria (11) by a blinded-to-treatment radiation oncologist during the radiotherapy course. The onset of ARD occurrence was considered the primary outcome while the secondary outcome was the intensity of ARD (i.e., the maximum RTOG score). Data were analyzed by using chi-square, and t-tests. The risk ratio (95% CI) was calculated and P-values of less than 0.05 were considered as the statistically significant difference. SPSS software version 21.0 (IBMCorp, Armonk, NY, USA) was used to perform statistical analyses.
4. Results
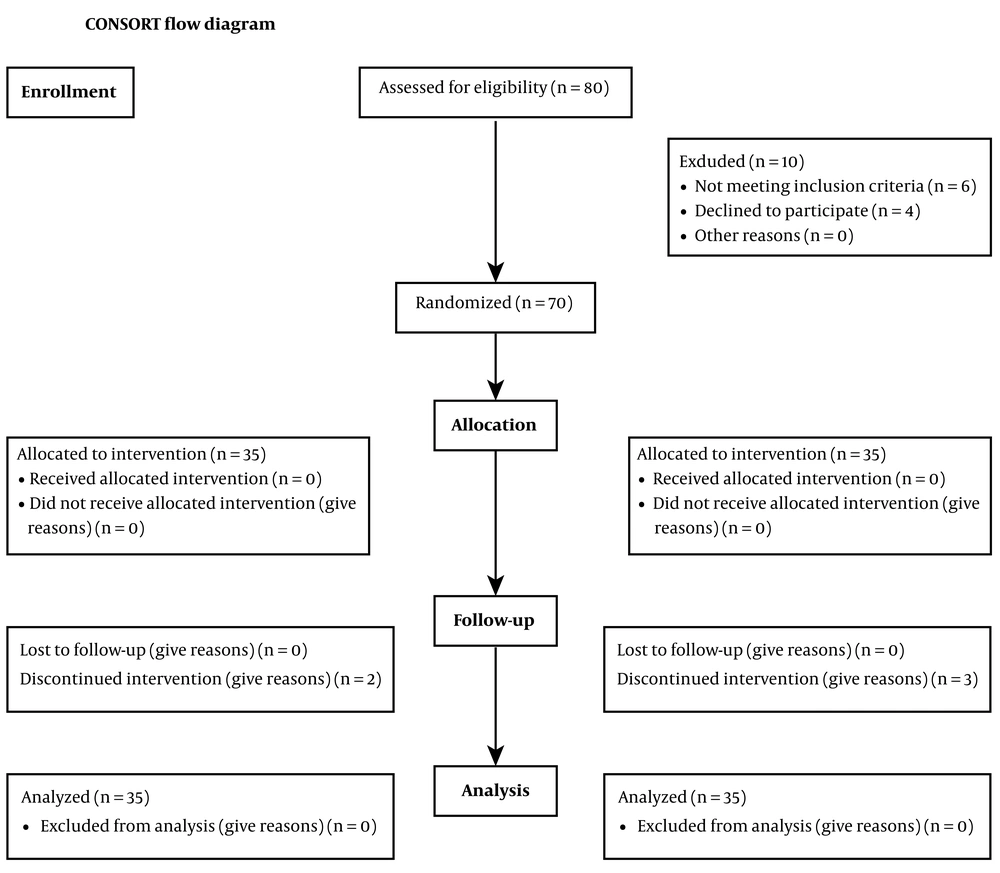
After the exclusion of patients who did not meet the eligibility criteria and who were denied participation in the study, finally, data from 70 patients were enrolled in the analysis. 35 patients were in the intervention group and 35 patients were in the control group (Figure 1).
Seventy patients were included, 35 in each arm. Patient and treatment characteristics are listed in Table 1. There was no significant difference between the two groups as far as age or BMI is concerned. The irradiation modalities such as target volumes, type of radiation and delivered dose were similar (see material and methods). The stage of disease, and the expression of ER, PR and HER2/neu receptors were not significantly different between the two groups.
| Variables | Mean ± SD |
|---|---|
| Age | |
| Case | 49.03 ± 12.25 |
| Control | 50.77 ± 10.61 |
| Height | |
| Case | 151.56 ± 26.83 |
| Control | 137.14 ± 47 |
| Weight | |
| Case | 70.81 ±17.29 |
| Control | 66.28 ± 23.62 |
| BMI | |
| Case | 28.98 ± 6.64 |
| Control | 28.80 ± 9.03 |
4.1. Acute Radiation-Induced Dermatitis
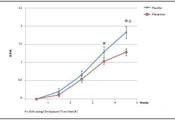
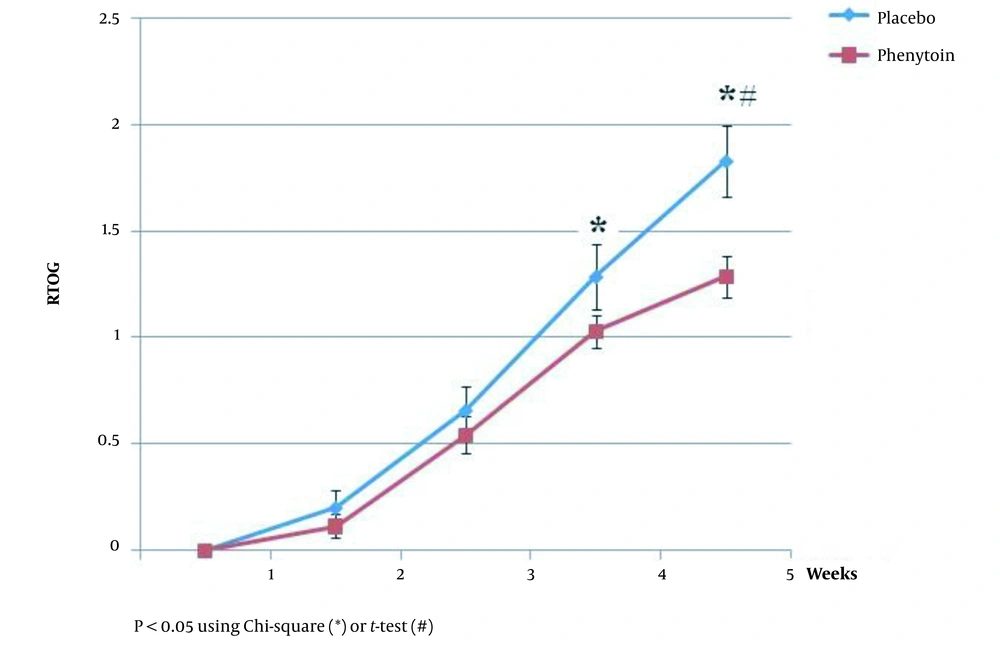
Although the mean ARD score significantly increased over the radiation period in both groups, it was significantly lower in the phenytoin group at the end of weeks 4 and 5 (P < 0.05) (Figure 2).
Thirty-four (97.1%) patients in the phenytoin group and 28(80%) patients in the placebo group did not develop severe dermatitis (RTOG ≥ 3), RR = 1.22 (95% CI: 1.02 - 1.045, P = 0.030). Six patients in the placebo group had to temporarily interrupt radiotherapy due to severe ARD symptoms whereas none did in the placebo group (Figure 3). Finally, no adverse drug reactions were observed whatever the groups. Patients’ ARD scores observed during the radiotherapy are presented in Table 2.
| RTOG Score (Week) | 0; No. (%) | 1; No. (%) | 2; No. (%) | 3; No. (%) | 4; No. (%) |
|---|---|---|---|---|---|
| 1 | 68 (97.1) | 2 (2.9) | - | - | - |
| 2 | 59 (84.3) | 10 (14.3) | 1 (1.4) | - | - |
| 3 | 28 (40) | 38 (54.3) | 4 (5.7) | - | - |
| 4 | 8 (11.4) | 45 (64.3) | 12 (17.1) | 5 (7.1) | - |
| 5 | 3 (4.3) | 32 (45.7) | 27 (38.6) | 4 (5.7) | 4 (5.7) |
5. Discussion
Although biological mediators of radiation skin toxicity are now better understood, there is still no effective therapy recommended for skin care. According to the guidelines for handling skin during radiation, for more than ten years, "washing with mild soap and lukewarm water" and the use of unscented, lanolin-free, water-based moisturizers, and avoidance of sun exposure have been recommended (12, 13).
Cancer centers have various standard care for controlling radiation dermatitis. A total of 21 sites were surveyed on radiation dermatitis standard care and none of them were the same. These included aloe vera, Aquaphor, udder cream, Radiaplex®, pulsed dye laser, corticosteroid creams, lidocaine cream, Silvadine®, and antibiotic ointment. There is no evidence that topical agents have therapeutic benefits (12, 14, 15). The use of topical corticosteroids has been evaluated in many studies with mixed results (12, 16). Barrier film spray (3M Cavilon Barrier Film) and prophylactic steroid cream (0.1% mometasone fuorate) make radiotherapy more tolerable for patients since they reduce inflammation and protect the skin barrier (17). There has been research showing that betamethasone-17-valerate cream, a potent corticosteroid, can be used by patients with breast cancer to reduce radiation dermatitis. However, the concern over skin integrity and potential side effects has prevented its widespread use.
The study by Di Franco et al. revealed that the combination of prophylactic topical hyaluronate and steroid therapy with Ixor® oral therapy (consisting of resveratrol, lycopene, vitamin C, and anthocyanins) cause a significant reduction in high-grade skin toxicity induced by radiation (18). Furthermore, the use of curcumin in combination with ginger and aloe vera has been shown increase wound healing (19, 20). Due to the anti-inflammatory, antioxidant, and cancer-fighting properties of curcumin have been evaluated as a potential therapy for over a decade. The results of a systematic review concluded that curcumin may benefit skin health. On the other hand, another study illustrated that there was no significant difference between oral curcumin and placebo in radiodermatitis severity. A variation in skin ratings and the application of broad criteria could not detect the therapeutic effect (21). Therefore, Vaughn et al. suggested additional clinical studies are needed to determine its effectiveness and mechanism (22, 23).
Although the advantage of trolamine is its tolerability, as well as its ability to moisturize the skin and alleviate local discomfort, it has not been proven that it is a topical skin radio-protective agent (24). Compared to trolamine, some controls demonstrated greater or similar effectiveness (2, 25-29). As the meta-analysis showed, there is no significant difference between controls and trolamine in order to prevent dermatitis due to radiation. According to the studies, chemotherapy and tamoxifen increase the skin reactions intensity in patients exposing radiation (30-33).
Low-level light therapy (LLLT) or vascular lasers have been made to control the symptoms while preventing or reducing radiodermatitis needs sufficient available evidence. LLLT has been demonstrated to possess bio-stimulating properties that facilitate tissue regeneration and healing faster, reduce inflammation, and prevent fibrosis in some preclinical and clinical studies. Radiation-induced telangiectasia has also been shown to be cured with pulsed dye laser treatment in late-onset radio-dermatitis (34).
However, skin wounds heal faster when phenytoin is used locally. No studies have been conducted on the effects of topical phenytoin on surgical wounds of patients. There are some evidence that treating skin ulcers with phenytoin diluted in NaCl 0.9% can avoid the formation of crust and burning that usually results when phenytoin powder is directly applied. Furthermore, in diabetics with recalcitrant neuropathic foot ulcers; phenytoin may improve wound healing.
Phenytoin provided better results therapeutically and cosmetically compared to cream (control). It is a low-cost drug with excellent pharmacoeconomic benefits, phenytoin 0.5% cream accelerates skin wound healing with good cutaneous tolerability and excellent cosmetic results in patients (35).
Various studies showing the prevention percentage of radiodermatitis in cancerous patients after applying different drug agents. Radiation Therapy Oncology Group grade 2 dermatitis was not improved in patients with head and neck cancer dressed in Silver Leaf nylon in Vavassis and colleague’s study (36). The study by Hemati et al. assayed the effect of silver sulfadiazine cream on the prevention of radiodermatitis in patients with breast cancer and illustrated that the incidence of RTOG grade 3 skin injuries was 3.9% in the fifth week, and 21.5% in the first week after radiotherapy ended (37) whereas, in our study, we did not have any patients who experienced RTOG grade 2 or higher. In 2001, Fiets et al. performed a randomized future trial of 74 patients with breast cancer undergoing radiotherapy. Randomly, they were either given Biafin or not. In comparison with patients without treatment, the Biafin group showed no improvement (38). However, Pommier et al. reported that Biafin (Trolamin) cream could reduce acute dermatitis induced by radiation (2). The results of Aygenc et al.’s study illustrated that pentoxifylline prophylaxis did not significantly affect the development of acute skin reactions (39). Although there are various studies on how to treat radiation-induced dermatitis, no gold standard is available for prevention or treatment. The effect of Recove® was evaluated on chest wall for dermatitis in patients undergoing breast radiotherapy. The study conducted by Abbaszade Marzbali et al. found that Recove® ointment significantly improved radiation-induced dermatitis of the chest wall in patients with breast cancer (40).
Acute radiodermatitis is an important side effect of radiotherapy. It might critically interfere with the treatment schedule and outcomes. Despite outstanding advances in radiotherapy techniques in recent decades, the standard radiotherapy for breast cancer treatment has not evolved much. Moreover, there is still significant room for improvement in the management of ARD. Phenytoin is an antiseizure drug with documented wound-healing properties. Its topical form has been used for decades to heal a wide variety of wounds including burn, excision, mucositis, and diabetic ulcers (10, 41-44). To our best knowledge, the present study is the first clinical trial that tested this agent for ARD. The double-blind nature of the present study provides a reliable assessment of ARD weekly. The applied RTOG scoring is a widely used and validated clinical scoring system for ARD (8).
Our data suggested that phenytoin significantly decreased the severity of ARD compared to the placebo, at least on weeks 4 and 5. It seemed that it also protected patients with breast cancer from severe ARD (RTOG score ≥ 3; Table 3) since no patient treated with phenytoin had to temporarily interrupt radiotherapy unlike 6 patients in the placebo group.
| RTOG Scoring Criteria | Skin Changes |
|---|---|
| 0 | No change over baseline |
| 1 | Follicular, faint or dull erythema, epilation, dry desquamation, decreased sweating |
| 2 | Tender or bright erythema patchy moist desquamation, moderate edema |
| 3 | Confluent, moist desquamation other than skin folds, pitting edema |
| 4 | Ulceration hemorrhage, necrosis |
Although there is no consensus on the management of ARD, different modalities have been tried to alleviate its signs and symptoms (7). In addition to the steroidal anti-inflammatory drugs, some non-steroidal antiin-flammatory agents (e.g., dexpanthenol, hyalorunic acid, aloe vera gel.) seemed to have a positive effect. Applying nonmedical agents (e.g., Biofie® topical emulsion), washing with soap and even with plain water turned beneficial. Although the exact mechanism of wound healing by phenytoin remains a debated issue, the present data suggested that it could significantly decrease or delay the occurrence of ARD. Phenytoin was suggested to stimulate fibroblast proliferation, collagen, and other extracellular matrix deposition and different growth factor production (10). In the pathophysiology of ARD, the production of free radicals leading to DNA damage and consequent inflammation and apoptosis have a critical role (1). It seems that topical phenytoin was able to modulate these effects and accelerate tissue repair.
5.1. Conclusions
The application of topical phenytoin seemed to be an effective agent in the alleviation of ARD severity. The present pilot study suggests that it could be an interesting ARD treatment option in patients with breast cancer. Phenytoin and other non-steroidal anti-inflammatory topical agents should now be compared in order to set the place of phenytoin.