1. Background
Worldwide, colon cancer is one of the most common causes of cancer- related mortality and morbidity (1).
During the last 25 years, the incidence of colorectal cancer has increased in Iran and based on the national registry, it is the third most common cancer in Iran following cancers of the breast and stomach with an annual incidence of more than 7100 cases (2).
Although colon cancers are still more common on the left side, many studies in recent years have shown a shift toward the right colon (1, 3).
The right (proximal) and left (distal) sides of the colon differ in their embryological origins and their tumors have different treatment responses and prognoses.
2. Objectives
In this retrospective study, we aimed at describing clinicopathologic characteristics of patients with colon cancer in two referral hospitals in Tehran, based on tumor sidedness.
3. Methods
3.1. Study Design and Setting
Data of the patients with colon cancer who had been treated from 2010 to 2020 at Imam Hossein University hospital and Milad General hospital in Tehran, Iran were retrospectively reviewed.
3.2. Eligibility Criteria
All patients who had been treated for colon cancer from 2010 to 2020 in the two referral hospitals and whose files contained the essential information entered the study (all available cases). Patients with a histologic diagnosis other than adenocarcinoma and patient with more than one synchronous tumor on both sides of the colon (based on the definition) were excluded. Besides, tumors located at the rectosigmoid junction were classified as rectal tumors and were excluded from the study.
3.3. Definition of Sidedness
Tumor location was determined mainly based on the surgical reports, as well as data obtained from colonoscopy and imaging.
Right-sided tumors were defined as tumors located in the cecum, ascending colon, hepatic flexure, and transverse colon, and left-sided tumors were defined as tumors located in the splenic flexure, descending colon, and sigmoid (1).
Tumor locations were determined for every year of the specified period and logistic regression analysis was performed to determine the possible change in the prevalence of tumor location during the study period.
3.4. Statistical Methods
The association between gender and age with the anatomical location was assessed by the chi-squared and/or Fisher exact tests. A P-value of less than 0.05 was considered significant. For non-parametrical assessment, the Mann-Whitney U test was used.
3.5. Ethical Approval Committee
Research ethics committee of Shahid Beheshti University of Medical Sciences.
4. Results
Among 1535 cases, 800 (52.1%) had left-sided and 735 (47.9%) had right-sided tumors. The most frequent tumor location was the sigmoid colon (549 cases, 35.8%) followed by the cecum (319 cases, 20.8%).
The patients included 849 (55.3%) males and 686 (44.7%) females with a mean age of 58.22 years (range: 22 - 89). The mean age of patients with left-sided and right-sided tumors was 58.37 (SD = 13.2) and 58.07 (SD = 13.8) years, respectively, which showed no difference between the two sides (P = 0.66).
Among patients from left-sided tumors, 381 (47.6%) were female, while on the right side, 305 (41.5%) patients were female and this difference was statistically significant (P = 0.016).
The most frequent grade was moderately differentiated (797 cases, 51.9%) followed by well-differentiated (539 cases, 35.1%) carcinomas.
The proportion of well-differentiated tumors was the same on both sides (271 (50.3%) on the left and 268 (49.7%) in right). There were more cases of moderately differentiated tumors on the left side (450 (56.3%)) compared to the right side (347 (47.2%)). However, the proportion of poorly differentiated tumors was much higher on the right side (120 (60.3%)) compared to the left side (79 (39.7%)), and this difference was statistically significant (P = 0.0001).
In patients who had undergone surgery, the most frequent T-stage was T3 (70.7%), followed by T4 (16.6%), T2 (12.2%), and T1 (0.6%). The most frequent N-stage was N0 (60.2%) followed by N1 (26.3%) and N2 (13.5%).
For analysis, we excluded the patients who had not undergone surgery (mainly metastatic patients).
The mean numbers of harvested and involved lymph nodes were 11.3 (range: 0 - 73, SD: 7.2) and 1.45 (range: 0 - 25, SD: 2.8), respectively.
The mean number of harvested lymph nodes on either side did not reach statistical significance (11.8 and 10.45 in right and left, respectively).
The ratio of involved nodes ranged from 0 - 1 (mean = 0.13, SD = 0.227). The ratio of involved nodes was not statistically different between the two sides (P = 0.382).
Among 1341 patients who had undergone surgery (87.4% of all patients), 55 (4.1%) had positive surgical margins, which was the same on both sides (27 on the right side and 28 on the left side).
In our study, 313 (20.4%) patients presented with de novo distant metastasis.
Among these 313 cases, 139 (44.4%) belonged to the right side primary and 174 (55.6%) to the left side and this difference was meaningful (P = 0.02).
Among patients with metastatic disease at presentation, the most frequent location of metastasis was the liver (55%) followed by the lung (18%) and peritoneal cavity (11%).
Among 136 patients who developed metachronous metastases, the most frequent location was the liver (45%) followed by the peritoneal cavity (17%). There was no difference between the locations of metastases on the two sides.
Data on lymphovascular and perineural invasion (LVI and PNI) were missing in 89 and 223 patients, respectively. The frequency of positive LVI and PNI was 41.6% and 33.9%, respectively in the total cohort of patients.
The number of patients with positive LVI on the right side was significantly higher than that of the left side (P = 0.002).
Both sides showed similar rates of positivity for PNI (P = 0.227).
Table 1 shows the frequency of colon cancer in different locations.
| Location | No. (%) |
|---|---|
| Sigmoid | 549 (35.8) |
| Cecum | 319 (20.8) |
| Ascending colon | 248 (16.2) |
| Descending colon | 194 (12.6) |
| Transverse colon | 134 (8.7) |
| Splenic flexure | 57 (3.7) |
| Hepatic flexure | 34 (2.2) |
| Total | 1535 (100) |
Frequency of Colon Cancer in Different Locations
Table 2 shows the demographics and clinical characteristics of patients with colon cancer between 2010 and 2020.
| Variable (Analyzed Cases b) | All Patients (N = 1535) | Tumor Location | P-Value | |
|---|---|---|---|---|
| Left | Right | |||
| Gender (1535) | ||||
| Male | 849 (55.3) | 419 (49.4) | 430 (50.6) | 0.009 |
| Female | 686 (44.7) | 381 (55.5) | 305 (44.5) | 0.009 |
| Mean age, y (1535) | 58.22 | 58.07 | 58.37 | 0.691 |
| Grade (1535) | 0.000 | |||
| Well diff | 539 (35.1) | 271 (50.3) | 268 (49.7) | |
| Mod diff | 797 (51.9) | 450 (56.3) | 347 (47.2) | |
| Poorly diff c | 199 (13) | 79 (39.7) | 120 (60.3) | |
| 2-year DFS d (1222) | 897 (73.4) | 489/624 (78.3) | 408/597 (68.3) | 0.001 |
| Involved surgical margin (1341) | 50 (4.1) | 28 (51) | 27 (49) | 0.231 |
| De novo metastasis (1529) | 313 (20.3) | 174 (55.6) | 139 (44.4) | 0.02 |
| Positive LVI (1446) | 601 (41.6) | 284 (47.3) | 317 (52.7) | 0.002 |
| Positive PNI (1312) | 445 (33.9) | 221 (32.9) | 224 (35.0) | 0.227 |
Demographics and Clinical Characteristics of Patients with Colon Cancer Between 2010 and 2020 a
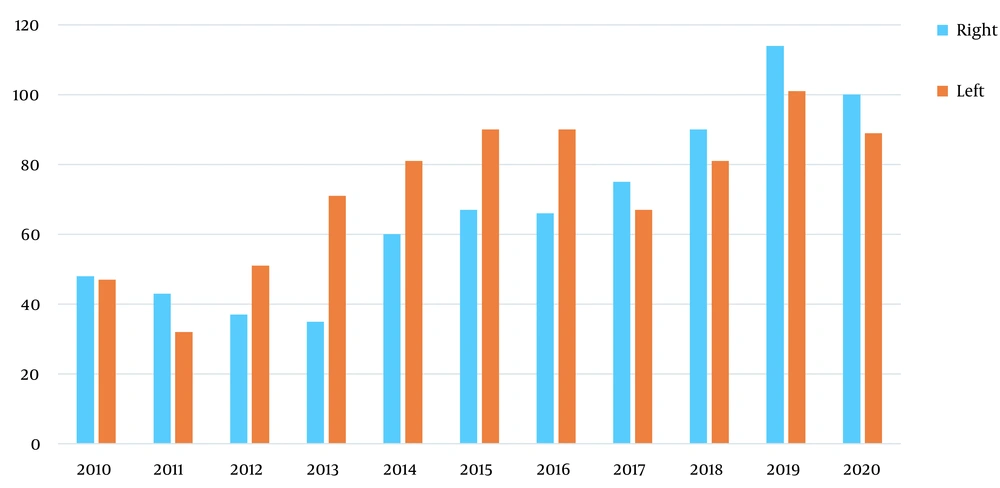
Figure 1 shows that although there were more cases of left-sided tumors compared to right-sided ones in total, there exists a trend toward shifting to the right side, which was statistically significant (P = 0.004).
Disease-free survival (DFS) was defined as the length of time after surgery to the first radiological or pathological evidence of recurrence. Patients with metastasis at presentation were excluded from the DFS analysis.
Two-year DFS for the patients with left-sided tumors was 78.3% compared to 68.3% for right-sided ones, and this difference was statistically significant (P = 0.01).
5. Discussion
Similar to many other studies, our study showed a change in the anatomical distribution of colon cancer with a rightward shift (1, 4).
The right and left sides of the colon differ in their embryological origins. The part extending from the cecum to the proximal two- thirds of the transverse colon originates from the midgut, and the distal third of the transverse colon to the upper anal canal derives from the hindgut and as a result, there are major differences between these two parts in terms of blood supply, innervation, lymphatic drainage, and lumen environment (3, 5).
Furthermore, probably due to differences in gut microbiota, the mucosal immunologic environment of the right colon is different from that of the left colon.
Eosinophils and intraepithelial T-cells are found in higher concentrations in the proximal colon, a fact that might explain the increased immune activity and the immunological response observed in tumors arising in the proximal colon (5).
Previous studies have shown mixed results regarding the rightward shift.
This conflicting result, apart from geographical distribution, race, number of evaluated cases, and other confounding factors, to some extent comes from the controversies in the exact definition of the right and left colon (anatomical location of the splenic flexure).
According to the widely accepted definition, right colon is defined as the part of the colon extending from the cecum to the splenic flexure (5, 6).
By considering the distal transverse colon as the place where the right and left sides of the colon are defined, the right colon includes the cecum, ascending colon, liver flexure, and transverse colon and the left colon includes splenic flexure, descending colon, and sigmoid colon.
In this study we followed this widely accepted definition and considered the splenic flexure as a part of the left colon, however, some studies have considered splenic flexure as a part of the right colon.
A study by Cucino et al. showed that between 1970 and 2000, there was a meaningful increase (16% - 22%) in the proportion of proximal lesions. They considered the splenic flexure as a part of the right colon, a fact that might have had an impact on the results (7).
Toyoda et al. reported that the proportions of right colon cancer among was consistently increased from 1974 to 2003 (from 21.5% to 25.6% in men, and from 28.2% to 36.8% in women). They also considered the splenic flexure as a part of the right colon (8).
Larsen and Bray by evaluating 102,882 cases from 1962 to 2006 reported that right-sided tumors continued to increase in both genders. They also considered the splenic flexure as a part of the right colon (9).
A study by Omranipour et al. on 157 Iranian patients from 1994 to 2009 did not show an increase in the proportions of right-sided cancers (there was only a slight and non-significant rightward shift in females). They considered the splenic flexure as a part of the left colon (1).
Hosseini et al. (10) analyzed the data on all colorectal cancer cases from 1970 to 2000 in Shiraz, Iran (based on the cancer registry), and demonstrated that the distribution of malignancy in both sides was almost equal with no significant changes during time. They did not mention their exact definition of sidedness.
Kashfi et al. (11) by assessing 258 patients in Iran from 2008 to 2013, found a higher occurrence of right-sided colon cancers (although statistically non-significant) and concluded that the pattern of the anatomical distribution of colorectal tumors has shifted toward the proximal colon. They did not mention their exact definition of sidedness.
Jalali and Jalali (12) in their study on 182 patients from 1990 to 2000 concluded that the site of colorectal carcinoma in Iran has shifted to distal, which is in the opposite direction of the majority of cases worldwide. They also did not clearly mention their exact definition of sidedness.
In the study of Ulanja et al. (6) on 163,980 patients, there were more cases of right-sided tumors than left-sided ones (52.3% and 47.7%, respectively). They considered the splenic flexure as a part of the left colon.
Recent data shows that localization of the tumor has important impacts on the early detection, presentation, molecular characteristics, management, and prognosis of patients with colon cancer (5, 11).
5.1. Age
Data on the relation between age and tumor sidedness is mixed. Available data shows that individuals with right-sided cancers tend to be older (6, 13-16). Omranipour et al. (1) did not find any difference between the ages of the patients on either side of the colon (mean ages of 55.4 and 54.5 years for the left and right sides, respectively).
We also did not find a difference between the ages of the patients on either side of the colon.
5.2. Gender
Similar to age, the data on the relation of gender and tumor sidedness is mixed. Some studies have suggested that the proportion of female patients was higher on the right side (3, 13, 17). Omranipour et al. (1) showed a slightly higher proportion of women diagnosed with left-sided colon cancer. Similar to this study, we also found that while the proportion of male patients was the same on two sides, female patients were more likely to develop left-sided disease.
5.3. Pathology and Stage
Right-sided tumors are more likely to have mucinous or signet ring cell histology and to be T3 or T4 at presentation (6, 13, 17).
A study by Ulanja et al. (6) on 163,980 patients showed that right-sided tumors were more likely to be N2 and T4, while there was no difference in the proportion of patients with de novo stage IV disease between the two sides.
Omranipour et al. (1) did not find any correlation between the tumor stage and its anatomical distribution.
In our study, among 199 cases of poorly diff tumors, 120 (60.3%) were located on the right side and 79 (39.7%) on the left side, and this difference was significant.
In contrast to several other studies that have reported a higher proportion of de novo metastatic patients in the right side primaries, our study demonstrated a higher proportion of de novo metastasis in the left-sided tumors and this difference reached the statistical significance (55.6% and 44.4% in left and right sides, respectively, P = 0.02).
5.4. Screening
Colonoscopy is considered the gold-standard modality for colorectal cancer screening. However, the sensitivity and specificity of this modality are lower in the proximal colon compared to the distal part (11, 18). One study on more than 4800 cases suggested that about 1 in 13 cases of colorectal carcinoma might be missed by colonoscopy. In that study, 11.3% of tumors in cecum, ascending colon and hepatic flexure and 11.7% in the transverse colon and splenic flexure had been missed by colonoscopy, while this figure for the left colon was 6% (19).
A study by Xiang et al. on 2093 patients with colorectal adenomas concluded that during colonoscopy, small and flat neoplastic lesions located in the right colon had a higher probability to be missed (20).
In case of the presence of right-sided shift, this issue could have important health-care consequences.
5.5. Presentation
Right-sided tumors more commonly present with iron deficiency anemia, intestinal perforation, and obstruction while changes in bowel habits and rectal bleeding are more common in left-sided tumors (3, 5).
Because of the larger diameter of the right colon, patients with proximal tumors generally have delayed onset of clinical symptoms, which can result in delayed diagnosis and more advanced disease at presentation (13).
5.6. Molecular Features
From a molecular point of view, right-sided and left-sided tumors vary considerably. While defects in mismatch repair genes (MMR) and mutations in KRAS and BRAF are more prevalent on the right side, left-side tumors are more commonly associated with mutations in p53 and NRAS (3, 17).
Differences in molecular mechanisms denote distinctions in tumorigenesis and progression between left and right colon tumors that have an important impact on making decisions for the management of each side (3, 21).
5.7. Chromosomal Instability
Eighty-five percent of colorectal cancers result from a type of genomic instability called chromosomal instability (CIN). This abnormality can result in the inactivation of tumor-suppressor genes and the activation of oncogenes (22).
As the main carcinogenesis mechanism of colon cancer, CIN differs by the location of the primary tumor: 75% of left-sided tumors and 30% of right-sided tumors are related to the CIN oncogenic pathway (3).
5.8. Microsatellite Instability
Microsatellite instability (MSI) results from impaired DNA mismatch repair (MMR) genes. Recent evidence indicates that most microsatellite instable (MSI-H) tumors originate from the right side of the colon (3, 23, 24).
5.9. RAS
RAS (KRAS, NRAS), which is activated and/or overexpressed in many cases of colon cancer, is a key downstream effector of EGFR (3). A recent meta-analysis demonstrated that the prevalence of KRAS mutations was significantly higher on the right side of the colon compared to the left side (46.3% and 35.8%, respectively, P < 0.0001) (25).
5.10. BRAF
BRAF mutation occurs in 2.5% - 20% of colorectal carcinomas (3).
Many studies have demonstrated that the BRAF mutation is more prevalent in right-side tumors compared to the left side (18.4% - 22.4% in right-sided and 1.3%-7.8% in left-sided tumors) (3, 25).
5.11. Consensus Molecular Subtypes (CMSs)
Four biological consensus molecular subtypes (CMSs) have been defined in colorectal carcinoma. CMS1 (microsatellite unstable) and CMS3 (metabolic) are more prevalent in right-sided malignancies (13).
CMS classification has potential clinical implications in patient management such as determining the type of chemotherapy in stage II or metastatic colorectal cancers. In addition, it can be used for designing novel agents that target specific pathways in each molecular subtype (26).
5.12. Patient Management
A growing body of evidence has denotes important differences between the right and left colon in terms of sensitivity to adjuvant, targeted and palliative therapies, and has suggested that these two sides should be regarded as two heterogeneous entities (3).
Several studies have shown different responses to specific adjuvant chemotherapy regimens between the two sides of the colon.
Elsaleh et al. (as cited by Shen et al.) reported that the survival benefits of adjuvant chemotherapy in stage III colon cancer were mainly observed in the right- sided tumors and the benefits on the left side were not significant (3).
Patients with MSI-H status (which is more common in the right side tumors) gain little benefit from 5-FU based adjuvant chemotherapy. This fact is of utmost importance, especially while making treatment decisions for completely resected patients with stage II disease (3, 5).
In patients with metastatic colorectal cancer (mCRC) receiving palliative therapy, the survival rate is different based on the location of the primary tumor (3).
Price et al. (27) by evaluating 2972 patients with mCRC reported that among the patients who only received basic supportive care, survival was worse for patients with right-sided tumors. When active therapy was offered, median OS of patients with right-sided and left-sided tumors was 18.2 months and 29.4 months, respectively (P < 0.001).
Besides, there exist reports indicating that mCRC patients with left- sided tumors are more likely to receive second, third, or fourth-line therapy compared to individuals with right-sided tumors (3).
Therefore, it seems that tumor site can be considered an independent prognostic factor of survival in patients with stage IV colon cancer (3).
One explanation for this could be the fact that patients with right-sided tumors generally have a higher number of adverse prognostic factors including poorly differentiated histology and advanced stage at presentation; and these factors can contribute to their poorer outcomes (3).
5.13. Anti-EGFR Therapy
Cetuximab has been shown to improve the outcome of mCRC patients who have RAS wild-type tumors. However, this improvement in survival is, to a great extent, location-dependent. Several studies have shown that right-sided tumors with wild-type KRAS gain less benefit from cetuximab (3, 5, 13).
5.14. Anti-angiogenic Therapy
The benefits of anti-angiogenic therapy appear to be site-dependent (3).
Previous studies (28) have reported that patients whose primary tumors were located in the rectum and sigmoid colon had significantly better outcomes when treated with bevacizumab, compared to patients with primary tumors in other locations.
5.15. Pattern of Metastasis
In mCRC, liver is the most common site of metastatic involvement, especially in left-side tumors. Peritoneal and lymph node metastases are more commonly associated with right-sided tumors (3, 13, 17).
5.16. Prognosis
A growing body of evidence has shown that the location of the primary tumor can affect the outcome in both adjuvant and metastatic settings (5). In the metastatic setting, patients with right-sided tumors generally have worse outcomes compared to those with left-sided tumors (13, 17).
However, this is still a matter of debate. In many studies survival rates following radical surgery have been reported to be similar between stages I-III of both sides of the colon (3). Results of the study of Benedix et al. on 17641 patients demonstrated that the 5-year DFS rates for right and left colon tumors were 73% and 74%, respectively (14).
Moritani et al. (29) analyzed the survival rates of 820 patients with stage I-III colon cancer and concluded that while there was no significant difference in survival between stages II and III, in patients with stage I disease, right-side tumors had a better 5-year DFS compared to left-side tumors (100% vs 95.2%, P = 0.034).
A meta-analysis of 66 published studies with more than 1.4 million patients with colon cancer demonstrated that regardless of stage, the absolute risk of death was 19% lower in left-sided tumors (5).
Zheng et al. (13) by evaluating 311,239 patients with colorectal cancer using the SEER database between 2006 and 2015 concluded that compared with right-sided tumors, patients with left-sided tumors showed superior OS in every stage.
In the study of Ulanja et al. (6) on 163,980 patients, individuals with left-sided tumors had better OS and cancer-specific survival (CSS) in stages I, III and IV, however, in patients with stage II disease, OS and CSS were better for right-sided tumors. For the entire cohort, the 3-year OS for right-sided and left-sided colon cancer was 67.6% and 72.5%, respectively (P < 0.001). Five-year overall survival was 58.1% for right-sided and 62.4% for left-sided tumors (P = 0.003). They argued that the older age of the patients with right-sided colon cancer with accompanying morbidities might -to some extent- explain the poorer overall survival observed in individuals with right sided cancer. Besides, they proposed that another reason for this worse survival might be related to screening; as left-sided cancers are more probable to be found at earlier stages using colonoscopy.
Hur et al. (16) by analyzing the data of 326,712 patients between 1996 and 2015 reported that compared to right-sided cases, patients with left-sided tumors had superior survival rates.
Sinicrope et al. (24) by analyzing the data of 2686 patients with resected stage III colon cancer who had been treated with a FOLFOX-based regimen showed that DFS was longer in left-sided tumors. In patients with an intact mismatch repair (MMR) system, DFS was superior for patients with left-sided tumors. In the group of patients with a deficient MMR system, better DFS was observed in right-sided tumors.
They concluded that in general, patients with left-sided tumors benefit more from FOLFOX, however, the results might be variable by considering the status of MMR proficiency.
In their study on 25377 patients, Mukkamalla et al. reported that right-sided tumors were associated with poorer outcomes, suggesting the role of underlying molecular or biological variants (30).
Our study, in line with the majority of available data, showed that patients with right-sided tumors had a worse 2-year DFS compared to the cases with left-sided tumors (68.3% vs 78.3%, P = 0.001).
5.17. Limitations
The retrospective nature of the study and missing data in the patients’ files could have impacts on the final results. Besides, because many patients discontinued their follow-ups 3 - 4 years following the treatment, providing data on overall survival was not possible.
5.18. Conclusions
Left and right colon tumors differ in molecular mechanisms involved in tumorigenesis. These differences in epidemiological, molecular, and histological parameters can have clinical implications. Right-sided tumors in general have more advanced stage, mucinous tumor, increased risk of peritoneal recurrence and overall, poorer prognosis compared to left-sided tumors.
Our study showed a right-sided shift in the last ten years, a fact that can have important clinical and epidemiological consequences. For instance, right-sided tumors are more likely to be missed in colonoscopy.
Based on the growing body of evidence, tumor sidedness should be acknowledged as an important epidemiological parameter with significant impacts on screening, tumorgenesis, response to treatment, and prognosis.