1. Background
Gastric cancer (GC) is considered one of the most fatal gastrointestinal (GI) malignancies which is mostly diagnosed in male patients than females (2.2 times) (1). Gastric Cancer is the fifth most frequent neoplasm accounting for a high incidence in Eastern and Central Asia and Latin America (2). Even though investigations on the molecular mechanisms of GC in the initial and progression stages have recently been developed (3), GC is still the third leading cause of cancer-related deaths worldwide (2). Over 7000 patients are diagnosed annually in Iran with gastric cancer, one of Iran's five most common cancers (4). The 5-year overall survival (OS) rate of GC patients is very dismal (less than 25%) and differs among assorted ethnicities and geographic regions (1). It has been illustrated that the chance of curing GC patients can be greater if the disease is diagnosed at early stages. Despite advanced screening and clinical approaches, the diagnosis and the therapy of GC are still challenging issues for therapists. Therefore, new diagnostic and therapeutic markers are required to pave the way for GC therapy (5). Recently, a large body of evidence has illustrated the prominent role of non-coding RNAs, particularly long non-coding RNAs (lncRNAs), in cancer progression and diagnosis (6). LncRNAs are an extensive family of non-coding RNAs with more than 200 nucleotides in length with little or no protein-coding capability. LncRNAs have significant roles in several cell biological processes such as cell proliferation, differentiation, apoptosis, and the epigenetic regulation of gene expression (7-9). Accumulating investigations have discovered that lncRNAs play considerable roles in tumor progression and development of multiple human cancers such as GC. It has been indicated that aberrant expression of lncRNAs deregulate important genes in cancer (6). LncRNAs may act as tumor suppressor genes and/or oncogenes which are completely cancer type-and tissue context-dependent (8). For instance, PANDAR is one of the tumor suppressors lncRNAs which is down-regulated in non-small cell lung cancer tissues. Overexpression of PANDAR has been shown to facilitate apoptosis and promote tumor cell progression (10). On the other hand, HOTAIR is one of the oncogenic lncRNAs that has been demonstrated to be up-regulated in a variety of aggressive tumor tissues. Overall, deregulation of lncRNAs is one of the main contributors on carcinogenesis (11). However, the exact underlying mechanisms of lncRNAs in GC are unknown yet. Investigation into lncRNA can extend our basic information about the modulatory mechanisms for better management of GC treatment and diagnosis. Recent investigations have revealed that exploring the potential novel biomarkers has a putative impact on the diagnosis and management of GC from the earliest appearance to the ultimate stages (12). Thymopoietin antisense transcript 1 (TMPO-AS1) is one of the lncRNAs that shows oncogenic activity in different kinds of human cancers. Thymopoietin antisense transcript 1 is transcribed in the antisense direction of the TMPO gene (13). Recent investigations have shown that TMPO-AS1 is up-regulated in some cancer tissues such as prostate cancer (14), non-small cell lung cancer (15), esophageal squamous cell carcinoma (16), cervical cancer (17), hormone-refractory breast cancer (18), osteosarcoma (19), and colorectal cancer (20). For instance, overexpression of TMPO-AS1 has been shown to significantly associate with tumorigenesis, whereas TMPO-AS1 depletion attenuated carcinogenesis and promoted apoptosis in cervical cancer cells (17).
Previous studies have shown that lncRNAs can exert their impact on the expression of protein-coding genes through their interaction with other molecules such as DNAs, miRNAs, transcriptional factors, etc. Androgen receptor (AR) and estrogen receptor (ER) are molecules that can be affected by lncRNAs (21). Huang et al. have shown that AR is effectively modulated by TMPO-AS1 in prostate cancer. In addition, a study in Japan on hormone-refractory breast cancer showed that TMPO-AS1 stabilizes ESR1 mRNA. By contrast, estrogen signaling is inhibited by TMPO-AS1 knockdown. Therefore, TMPO-AS1 is affected by some sex hormones receptors such as AR and ER in prostate and breast cancers, respectively (14, 18). Gastric cancer is one of the male-predominant cancers, and encoding the factors that contribute to this sex-related disparity can unveil novel GC tumorigenesis pathways (22).
The association between TMPO-AS1 and important cell signaling pathways such as RB-E2F has been reported in several cancers, including lung adenocarcinoma (23), bladder cancer (24), osteosarcoma (25), and gallbladder carcinoma (26). On the other hand, inactivation of the RB-E2F signaling pathway and E2F1 overexpression can induce gastric cancer tumorigenesis (27, 28). Therefore, considering the high prevalence of GC in Iran and that both GC and TMPO-AS1 are effective in the same cancer cell signaling pathways, TMPO-AS1 expression levels we decided to investigate in Iranian patients with GC.
2. Objectives
To reveal if the lncRNA TMPO-AS1 deregulates in GC, we performed in silico and experimental analyses of long non-coding TMPO-AS1 expression in adjacent nonmalignant tissues and cancerous tissues of Iranian patients with GC.
3. Methods
3.1. Patients and Sampling
In the present study, 40 gastric tumor samples and 40 margin noncancerous counterparts were collected from 40 (21 males and 19 females) patients with GC whose disease (GC) was confirmed by the physicians and pathologists at Imam Reza Hospital, Tabriz University of Medical Sciences, Tabriz, Iran. Due to tumor infiltration possibility in marginal tissues, the samples were examined by pathologists and then included in the study. The exclusion of patients with a history of receiving clinical treatments such as radiotherapy, chemotherapy, or immunotherapy was considered inevitable for the study. The ethical committee of Tabriz University confirmed the protocol of this study (N: IR.TABRIZU.REC.1399.009). The samples were frozen in liquid nitrogen immediately after samples were taken during surgery to prevent RNA degradation. The clinicopathological characteristics of samples are reported in Table 1. Briefly, 52.5% of patients were male while 47.5% were female. Of the patients, 47.5% were identified as stage I or II, and 52.5% as stage III or IV.
| Characteristic | Number of GC Patients; No. (%) |
|---|---|
| All patients | 40 (100) |
| Age (y) | |
| < 60 | 34 (85) |
| ≥ 60 | 6 (15) |
| Gender | |
| Female | 19 (47.5) |
| Male | 21 (52.5) |
| Smoking | |
| No | 28 (70) |
| Yes | 12 (30) |
| Negative | 25 (62.5) |
| Positive | 15 (37.5) |
| Negative | 35 (87.5) |
| Positive | 5 (12.5) |
| Negative | 19 (47.5) |
| Positive | 21 (52.5) |
| Stage | |
| I + II | 19 (47.5) |
| III + IV | 21 (52.5) |
| Poor | 11 (27.5) |
| Moderate | 16 (40) |
| Good | 13 (32.5) |
3.2. RNA Isolation and cDNA Synthesis
Total RNA was extracted by TRIzol isolation solution (GeneAll Biotechnology, Seoul, Korea) using the guanidinium-isothiocyanate method. The quality evaluation of extracted RNAs was verified via Nano-drop (Thermo Fisher Scientific, USA) spectrophotometer by measuring absorption at 260/280 nm. Then, the isolated RNAs were reverse-transcribed to complementary DNA (cDNA) using cDNA synthesis kits (Biofact, Seoul, South Korea) according to the manufacture’s instrument.
3.3. Quantitative Real-time Polymerase Chain Reaction
The TMPO-AS1 expression levels in patients' samples were determined by polymerase chain reaction (qRT-PCR) analysis (Roche real-time PCR Light Cycler 96 device). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was considered the internal control gene. The sequences of gene-specific primers for TMPO-AS1 and GAPDH, which had blasted on the NCBI website, are summarized in Table 2. The total volume was 20 μL including 10 μL SYBR Green, 1 μL primer (0.2 μM concentration of each primer), 2 ng/μL cDNA and the rest DEPC water. Thermal cycling conditions composed of an activation step for 15 min at 95°C followed by 40 cycles, including a denaturation step for 10 seconds at 95°C, 1 min at 60°C (TMPO-AS1), at 59°C (GAPDH) for annealing, and 20 seconds at 72°C for the extension. The evaluation of the lncRNA expression was conducted by ΔΔCT formula and relative quantification as follow:
| Gene | Primer Sequence | Product Size (bp) |
|---|---|---|
| TMPO-AS1 | 139 | |
| Forward | 5′-AGACGCCGATAAGGGACAG-3′ | |
| Reverse | 5′-AGCCAAGGGTCCTCACA-3′ | |
| GAPDH | 166 | |
| Forward | 5′-CAAGATCATCAGCAATGCCTCC-3′ | |
| Reverse | 5′-GCCATCACGCCACAGTTTCC-3′ |
3.4. Bioinformatics Analysis of TCGA Dataset
The Cancer Genome Atlas data were retrieved and analyzed via GEPIA (http://gepia.cancer-pku.cn/) and TANRIC (https://ibl.mdanderson.org/tanric/_design/basic/main.html) online tools. Thymopoietin antisense transcript 1 expression and corresponding disease-free survival (DFS) and overall survival (OS) were analyzed by GEPIA online tool. Subgroup analyses of the gene expression were conducted via TANRIC online software. Selections were done by two criteria Fold change (FC) > 2 and P-value < 0.05.
3.5. Statistical Analysis
All the data are presented as mean and SD. The study employed student t-test and one-way ANOVA to analyze the obtained data. The chi-square test (Spearman) was also conducted to evaluate the association between lncRNA expression level and clinicopathological characteristics. A receiver operating characteristic (ROC) curve was plotted to assess the diagnostic value. All the statistical analyses were done via SPSS v23.0 software (IBM Corp., Armonk, NY, USA). A P-value < 0.05 was considered significant.
4. Results
4.1. TMPO-AS1 Expression in GC Patients
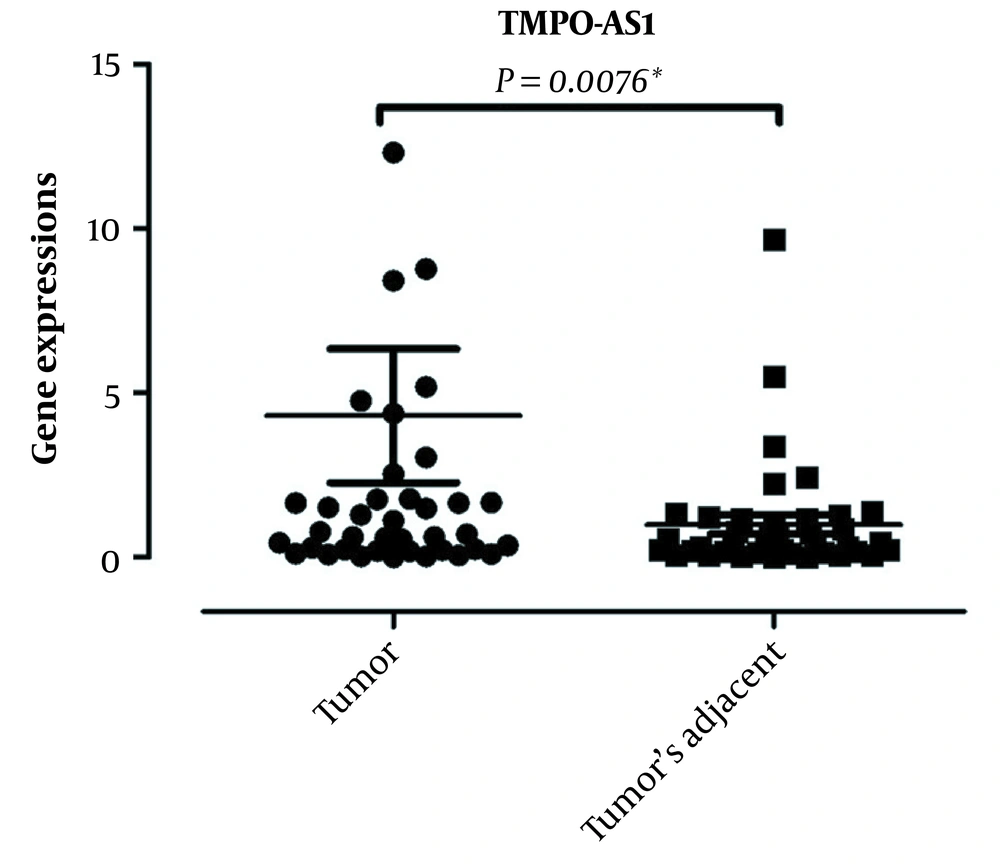
The lncRNA TMPO-AS1 expression was evaluated in 40 tissues of GC patients in comparison to noncancerous counterparts. The data demonstrated that TMPO-AS1 was overexpressed in the malignant tissues (median: 4.8, P: 0.0076). The results are presented in Figure 1.
Additionally, the patients were divided into two groups (high expression and low expression) according to the Median expression of TMPO-AS1. The results revealed no significant correlation between TMPO-AS1 expression and demographic as well as clinicopathological data including age, gender, smoking, lymph node metastasis, distant metastasis, Helicobacter pylori infection, various stages, and differentiation. The results are summarized in Table 3.
| Parameters | TMPO-AS1 | P-Value | |
|---|---|---|---|
| Low Expression | High Expression | ||
| Age (y) | 0.182 | ||
| < 60 | 15 | 19 | |
| ≥ 60 | 5 | 1 | |
| Gender | 0.113 | ||
| Female | 12 | 7 | |
| male | 8 | 13 | |
| Smoking | 0.49 | ||
| No | 13 | 15 | |
| Yes | 7 | 5 | |
| Lymph node metastasis | 0.744 | ||
| Negative | 13 | 12 | |
| Positive | 7 | 8 | |
| Distant metastasis | 0.633 | ||
| Negative | 17 | 18 | |
| Positive | 3 | 2 | |
| Helicobacter pylori infection | 0.113 | ||
| Negative | 12 | 7 | |
| Positive | 8 | 13 | |
| Stage | 0.113 | ||
| I + II | 12 | 7 | |
| III + IV | 8 | 13 | |
| Differentiation | |||
| Poor | 7 | 4 | |
| Moderate | 7 | 9 | 0.31 |
| Good | 6 | 7 | 0.392 |
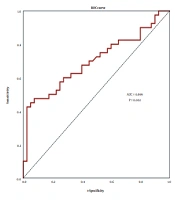
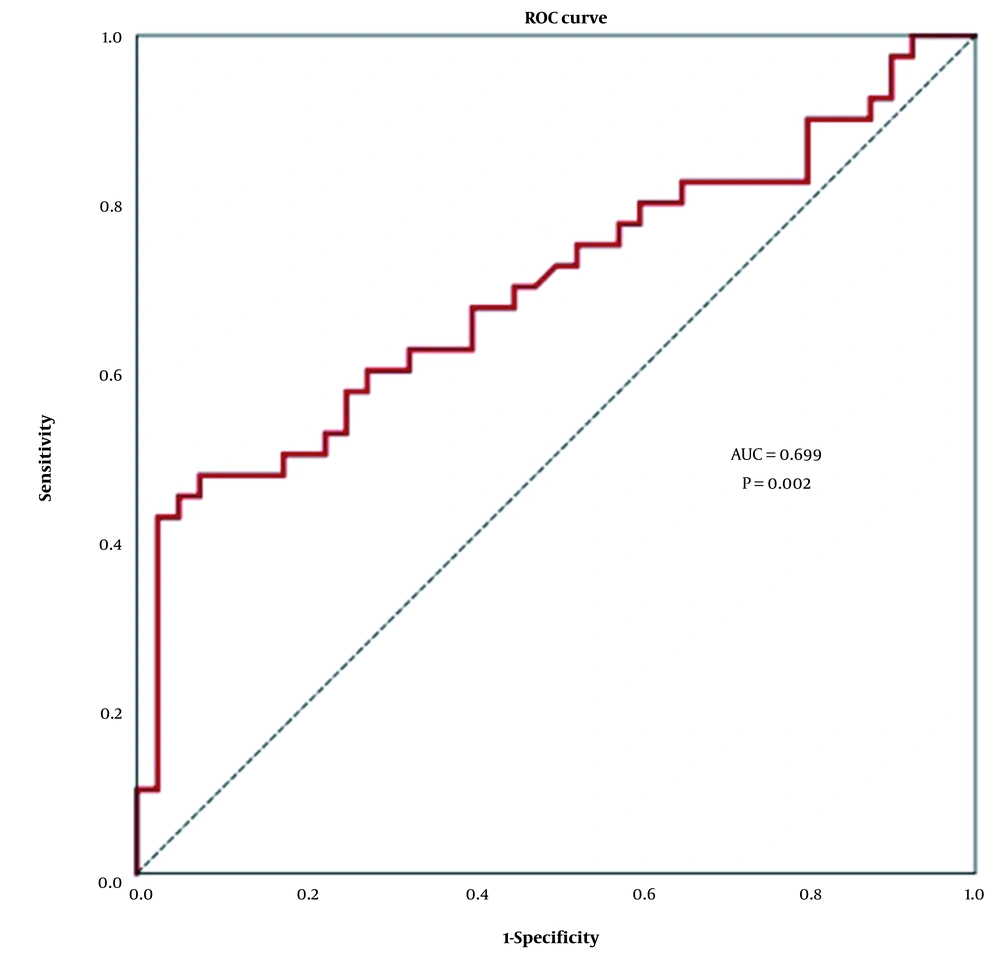
Furthermore, the ROC curve indicated a promising diagnostic capability for TMPO-AS1 in patients with GC. The results showed that the area under the ROC curve (AUC) was 0.699 (99% confidence interval 0.546 - 0.852; P = 0.002) for differentiating GC from normal tissues (Figure 2).
4.2. TCGA Data of TMPO-AS1 for Patients with GC
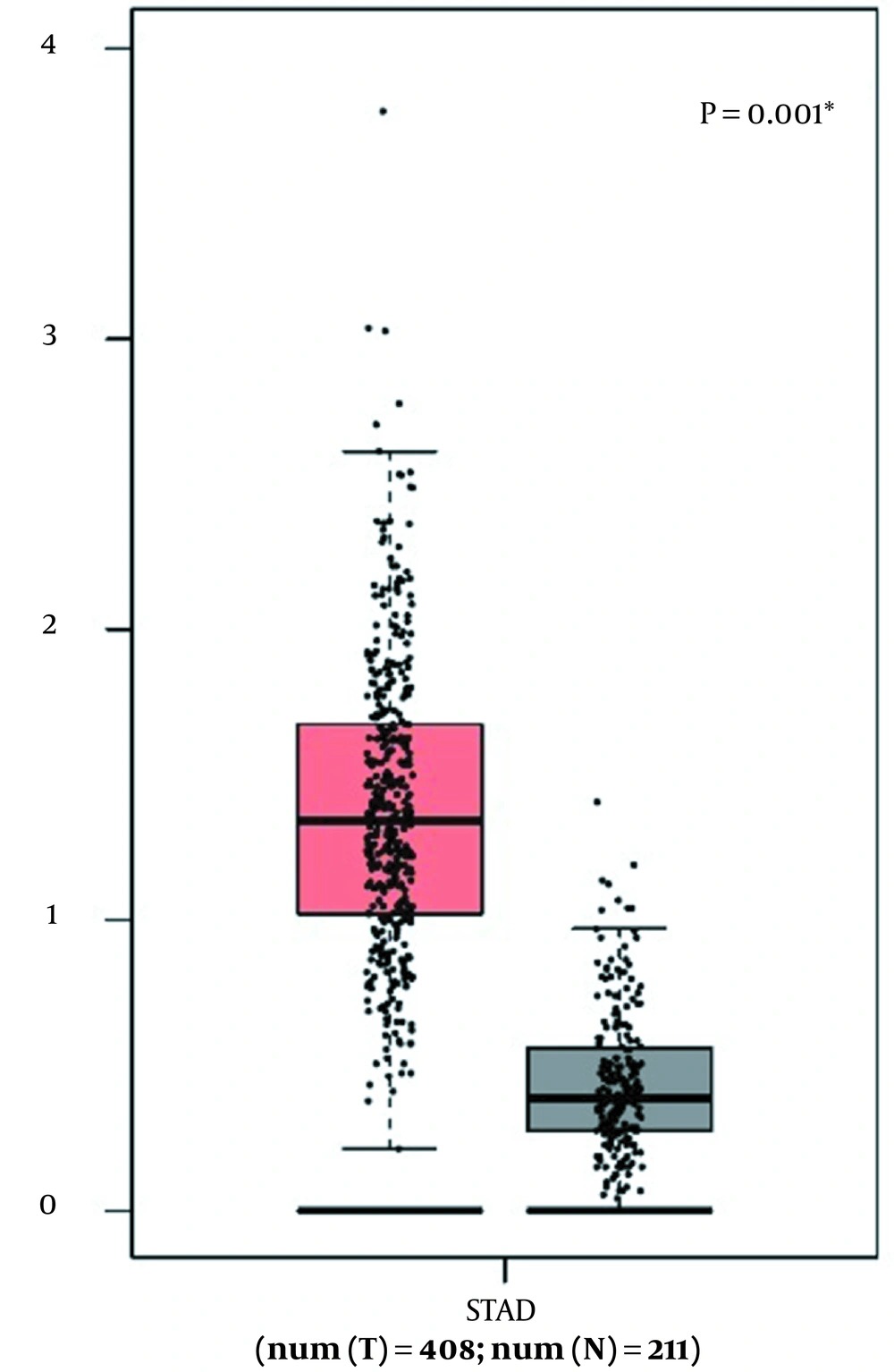
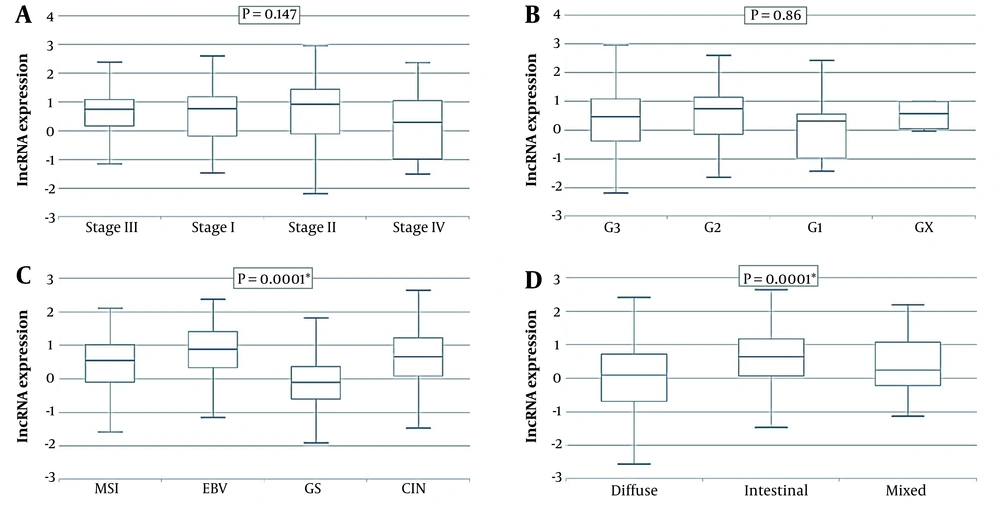
The Cancer Genome Atlas (TCGA) data of 408 GC patients’ tissues and 211 normal counterparts were retrieved and analyzed via GEPIA and TANRIC online tools. The data demonstrated that TMPO-AS1 was upregulated in GC tissues in comparison to normal counterparts (P = 0.001) (Figure 3).
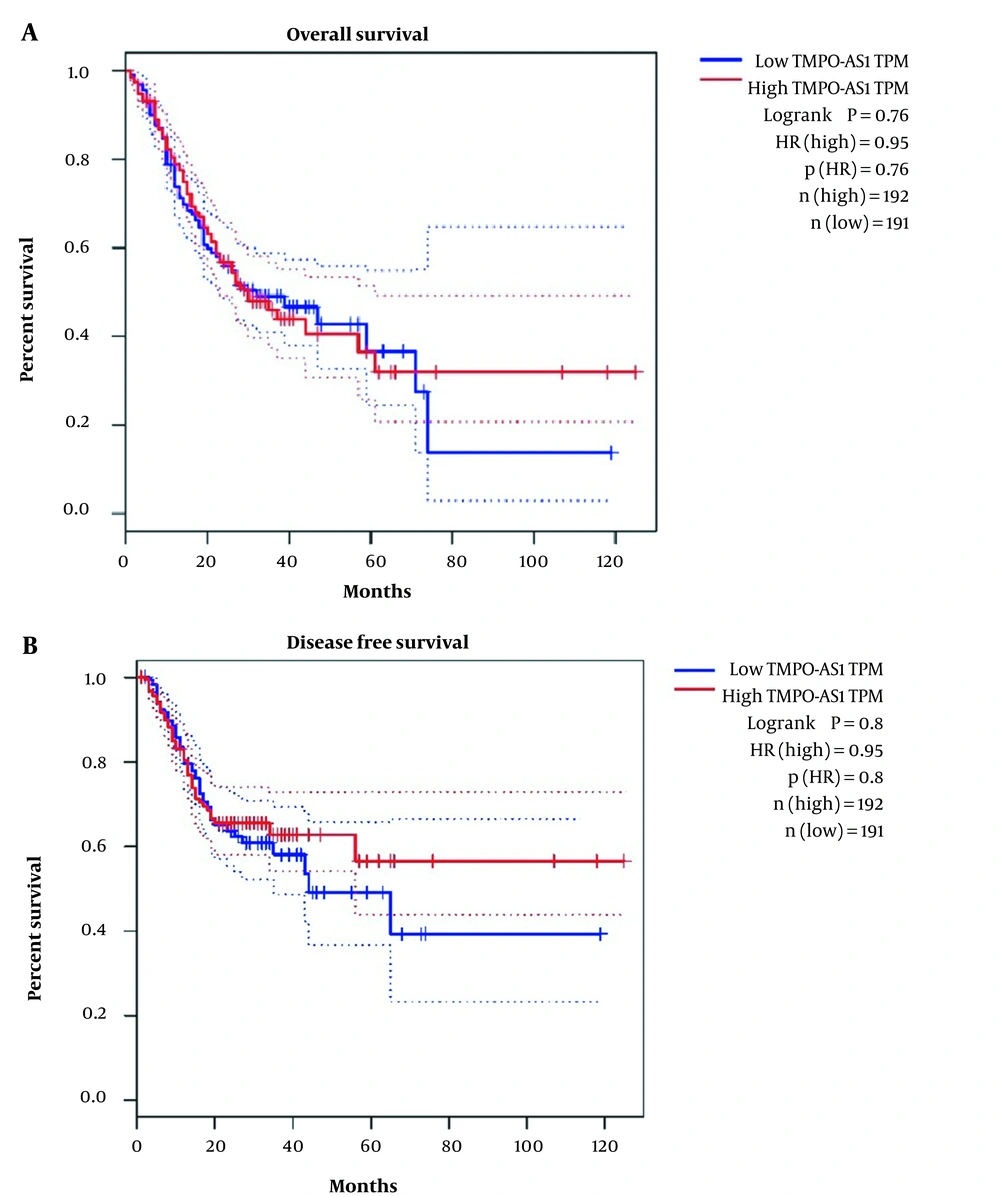
Furthermore, it was demonstrated that TMPO-AS1 expression did not correlate with tumor stage and grade (P = 0.147, P = 0.86, respectively), whereas it correlated with molecular and Lauren classification (P = 0.0001). The data are presented in Figure 4. Moreover, Kaplan-Meier curve analyses did not show any significant correlation between TMPO-AS1 expression and overall survival (OS) (P = 0.76) as well as disease-free survival (DFS) (P = 0.8) in these patients (Figure 5).
5. Discussion
Gastric cancer is the foremost cause of cancer-related death in Iranian men and women (4). Therefore, it is necessary to improve diagnosis and prognosis and ultimately treatment of patients with GC by finding new molecular biomarkers effective in tumorigenesis and tumor progression for patients with GC (29). Thymopoietin antisense transcript 1 is one of the oncogenic lncRNAs in several cancers that is involved in tumorigenesis and associated with poor prognosis. Investigations have previously shown that TMPO-AS1 overexpression results in increased cancer cell proliferation. Conversely, the knockdown of TMPO-AS1 results in enhanced cancer cell apoptosis and attenuates cell proliferation in some cancer types such as osteosarcoma and breast cancer (19, 30). Thymopoietin antisense transcript 1 deregulation can activate downstream oncogenes’ expression by functioning as a competing endogenous RNA (ceRNA) and inhibiting miRNA expression in multiple cancers (13).
In this study, we demonstrated overexpression of TMPO-AS1 in tumor tissues of GC patients relative to noncancerous adjacent tissues, which, in turn, is related to the prognosis of GC patients. Also, up-regulated TMPO-AS1 expression in tumor tissues compared with adjacent tissues has been revealed by TCGA analysis. Furthermore, ROC curve analysis confirmed that TMPO-AS1 expression level can significantly discriminate gastric tumors from non-tumor tissues. These three different results reinforce each other and confirm the importance of studying the potential role of TMPO-AS1 as a prognostic, diagnostic or therapeutic target in GC patients. Consistent with these findings, in China, Sun and Han observed that TMPO-AS1 overexpression promotes cell migration and invasion in GC. Some clinicopathological features, including age, gender, lymph node invasion, and differentiation, were not significantly associated with TMPO-AS1 expression (31), in agreement with the results of the present study. Although Sun and Han reported an association of TMPO-AS1 expression with tumor size and clinical stage, we did not observe any significant association with these clinicopathological characteristics in both in silico and experimental analyses.
For the first time in 1983, Tokunaga et al. demonstrated the association between sex hormone receptors and GC (32). It has been postulated that high GC incidence rate in men compared to women is due to estrogen effects. However, there are some findings supporting or refuting this postulation (33). The current study showed no significant correlation between TMPO-AS1 expression and patients' gender.
These different results may be associated with common study limitations, including sampling errors and the limited number, heterogeneity, and various genetic backgrounds of samples. Tumor heterogeneity and genomic complexities of GC could be related to these results. Since cancer is a multifactorial disease and many coding and non-coding genes affect it by disrupting different molecular signaling pathways, each cancer sample requires more research to find unique changes in pathogenesis (29, 34).
LncRNAs have been identified as a class of noncoding RNAs that have various modulatory roles in several biological processes. The ectopic expression of lncRNAs has been explored as a potential diagnostic and prognostic biomarker in GC (35). Previous investigations have cleared that TMPO-AS1 was an important cell proliferation modulator and could be considered as a potential diagnostic and prognostic biomarker in multiple cancer types (13). These studies agree with the present study's results, as according to our findings, the ROC curve showed TMPO-AS1 could significantly distinguish GC and non-cancerous tissue samples.
5.1. Conclusions
Taken together, we demonstrated that TMPO-AS1 is significantly more expressed in cancerous tissues than in the marginal noncancerous tissues in GC patients. Based on the preservative role of ER and AR in the etiology of GC, and the relationship between TMPO-AS1 and sex hormone signaling pathways, further investigations on the possible roles of TMPO-AS1 in the sex hormone signaling pathways in GC are required. Furthermore, based on the ROC curve, TMPO-AS1 expression levels could differentiate GC tumors from non-tumor tissues, highlighting its biomarker potency. However, further investigations are needed to confirm this result.