1. Background
COVID-19 has imposed a heavy burden on the healthcare systems around the world since the last month of 2019 and early months of 2020, with over 532 million cases and 6,312,535 deaths reported in May 2022 (1). Most countries have implemented widespread anti-epidemic measures but are still struggling to decrease transmission rates at high levels (2).
Vaccination strategy has reduced the COVID-19 occurrence rate, and the highest drop rate has been recorded for those aged over 65 years. In addition, it has reduced the adverse consequences and death rate of the disease, ICU admission, and non-ICU admission (3).
Medical experts believe that the pandemic may last several months or even years. Patients with cancer have been at an increased risk of infection during this period and; therefore, providing them with specialized healthcare services is essential (3, 4).
Recent data have suggested that patients with cancer are more prone to develop severe infections and have a higher rate of serious complications and mortality from COVID-19 (5). To minimize the medical staff workload, reduce the number of hospital visits, and decrease the risk of iatrogenic exposure to COVID-19, it might be possible to omit, defer or re-schedule some treatment modalities for carefully selected patients (5, 6).
More effective communications should be established between healthcare teams and multidisciplinary decision-making authorities based on both the stage of the disease and the treatment type (7).
Two strategies that could be used at the peak of COVID-19 to decrease the risk to patients’ health and preserve healthcare resources are adopting deferred surgery and initiating neoadjuvant therapy (8).
During this period, access to medical facilities might be restricted, which results in interruptions in the diagnosis and treatment. To address these obstacles, practical protocols should be designed and implemented considering the fact that some recommendations from international guidelines are not applicable in many parts of the country.
2. Objectives
This study aimed to provide recommendations for managing patients with prostate cancer properly during the COVID-19 pandemic based on our available nationwide resources.
3. Methods
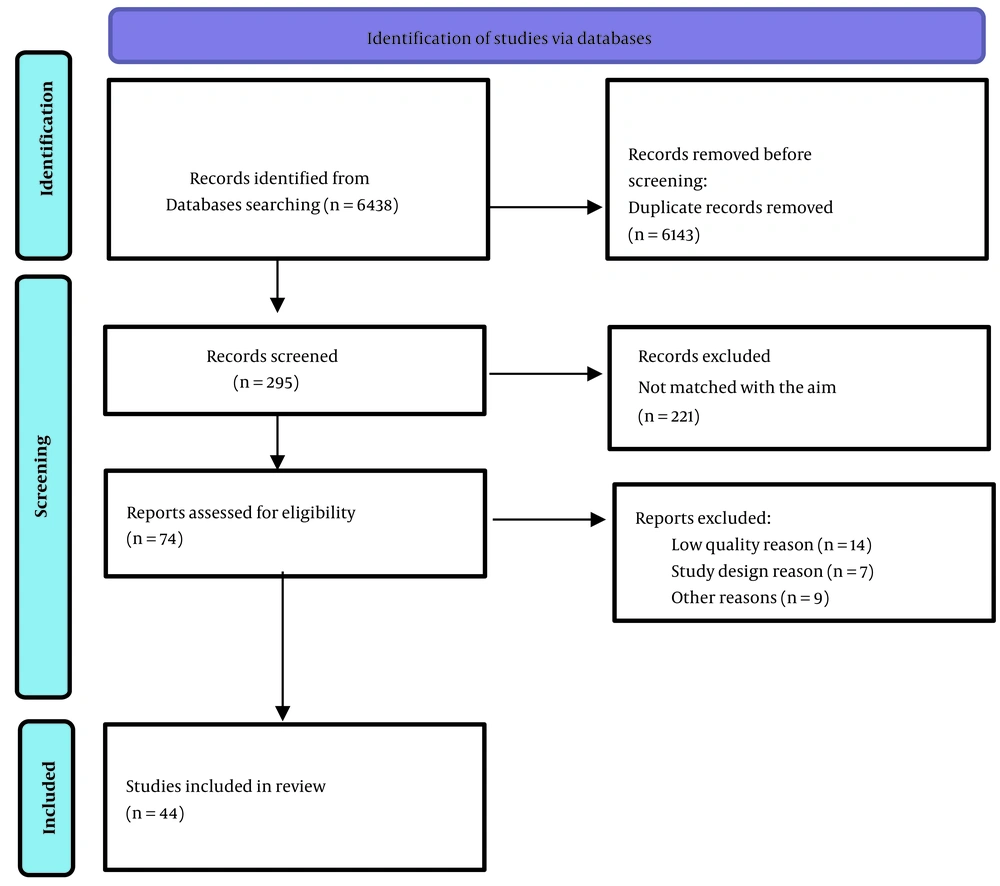
This study was a systematic review conducted from 2019 to 2022, which was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences with the code: IR.SBMU.RETECH.REC.1400.935. We defined participants (all articles in the COVID-19 era about diagnosis and management of prostate cancer), interventions (all changes in prostate cancer diagnosis and management protocols), comparisons (if available to the time before the pandemics) and outcomes (if available about survival or morbidity). A web search was performed using the keywords: “Diagnosis”, “Biopsy”, “Management”, “Radical Prostatectomy”, “Radiotherapy”, “Hormone Ablation Therapy”, “Chemotherapy”, “Prostate Cancer”, and “COVID-19”. The visited databases were PubMed, Scopus, Web of Sciences, Google Scholar, and Scientific Information Database. The search was performed by two researchers independently and supervised by the third one. The researchers assessed the retrieved articles (n = 6438), and then the useful articles were selected (n = 74). Then the quality of the articles was evaluated by a team of experts on methodology. The review was completed by selecting a total of 44 studies. Figure 1 displays the selection process. By following items were included in the study: Relevant studies in English or in other languages with an English abstract, international guidelines, original research studies, randomized controlled clinical trials, as well as proper observational, cohort, and case-control studies.
Preferred reporting items for a systematic review and meta-analyses (PRISMA) flow diagram (9).
Data quality control and scoring were performed using the Consolidated Standards of Reporting Trials (CONSORT) checklist and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist.
4. Recommendations
4.1. Diagnosis
A definite diagnosis of prostate cancer should be usually confirmed by histopathology confirmation. Taking into account the facts that the treatment of prostate cancer does not reduce its mortality in low-risk patients and the complications resulting from prostate cancer management reduce the patients' quality of life (e.g., urinary incontinence and erectile dysfunction), implementing a risk assessment method seems essential to avoid unnecessary prostate biopsies (10, 11).
While the prostate biopsy is indicated in the case of an abnormal digital rectal exam (DRE), measuring the prostate-specific antigen (PSA) is usually the preferred method for predicting the presence of prostate cancer (12).
Patients with higher PSA levels are more likely to harbor a cancerous focus in their prostate; therefore, there is no optimal PSA threshold for detecting prostate cancer (13). Patients with a slight rise of PSA should be re-evaluated one month later with a repeat test.
Empiric antibiotics should not be prescribed for asymptomatic men with a slight PSA elevation (14). Free to total PSA ratio is a helpful method when the PSA level is between 4 - 10 ng/dL (15). In addition, risk calculators are helpful tools for stratification of the risk of developing prostate cancer. However, none of them is an ideal method (16).
Multiparametric magnetic resonance imaging (MP-MRI) is a modality with a high sensitivity but low specificity in detecting prostate cancer. It can help urologists avoid 30% of all prostate biopsies. If only patients with a PI-RADS ≥ 3 (prostate imaging reporting and data system) are scheduled for a biopsy, however, 11% of all grade ≥ 2 cancers [based on the International Society of Urological Pathology (ISUP)] are missed (17).
Combining PSA density with PI-RADS score is a valuable method in making decisions to perform a prostate biopsy. If PSA density in patients with negative MRI (PI-RADS = 1 - 2) is less than 15 ng/mL, the risk of developing prostate cancer would be less than 10%; if PSA density is more than 15 ng/mL, however, this value increases to 27 - 40% (18-20).
MP-MRI should not be considered a screening tool because of its low specificity in very low-risk cases (21). Also, prostate cancer screening should not be performed in asymptomatic men until the COVID-19 pandemic dwindles because even a 6 - 12 months delay in the diagnosis does not significantly increase the mortality or morbidity of prostate cancer (22-24).
4.2. Prostate Biopsy
Prostate biopsy is performed by trans-perineal or trans-rectal ultrasound (TRUS) guided methods that have similar detection rates. Oral or intravenous antibiotics are recommended in this regard. For a trans-rectal biopsy, prophylaxis with a single dose of quinolone such as ciprofloxacin is sufficient; however, overuse and misuse of fluoroquinolones have increased fluoroquinolone resistance. Therefore, a targeted therapy in case of fluoroquinolone resistance or augmented prophylaxis (i.e., combination of two or more different classes of antibiotics) is recommended (25).
Rectal disinfection with povidone-iodine might be considered in TRUS biopsy (26). Unfortunately, the post-biopsy infection has increased because of the increased resistance to quinolone (27). Factors that increase the risk of resistance to quinolones include a history of TRUS biopsy, indwelling catheter, a urinary infection, or hospital admission within the prior six months. TRUS-guided biopsy with prior rectal swab culture should be performed in patients with any of these risk factors, or the trans-perineal approach should be employed in this regard (15). A single dose of intravenous cephazolin should be used as the prophylactic antibiotic for dealing with patients whose trans-perineal biopsy has been scheduled (28).
European association of urology (EAU) and American urological association (AUA) guidelines have a similar approach to diagnosing prostate cancer during COVID-19. According to these guidelines, if PSA or DRE suggests local prostate cancer, it is better to delay the biopsy for up to 3 months, preferably with by using the trans-perineal method. However, if the locally advanced disease is detected by DRE or the patient is symptomatic, a prostate biopsy should be scheduled within six weeks (29).
4.3. Intra-operative Care
Given the possibility of a false-negative test, personal protective equipment should be designed and implemented for all healthcare workers (30, 31).
4.4. Post-operative Care
The patients are still at the risk of the severe COVID-19 following the operation; therefore, post-operative precautions should be taken. In patients with prostate cancer, most prostatectomies can be safely delayed and the decisions making for high-risk patients should be individualized depending on factors such as age, disease stage, and comorbidities (32).
4.5. Local and Locally Advanced Disease
During the COVID-19 pandemic, it is inevitable to provide necessary support for the healthcare system and preserve hospital resources without compromising patient outcomes.
In patients with low-risk prostate cancer, no staging tests and confirmatory biopsies or treatments are recommended because a deferred treatment for this group of patients is safe for up to 6 - 12 months (5, 6, 8). In patients with intermediate-risk prostate cancer who are a candidate for radical prostatectomy, it is wise to postpone the surgery until the COVID-19 pandemic subsidies (5, 6). As for patients with high-risk prostate cancer and since the surgery is an essential part of multimodality treatment, two options are available during the COVID-19 pandemic (33): (1) performing deferred surgery and staging until safety is assured; (2) performing radiotherapy and androgen deprivation therapy (ADT) (10).
Data from John Hopkins University suggested that deferred surgery for six months fail to exert adverse effects on the outcome of patients with high-risk or unfavorable intermediate-risk of prostate cancer (24, 34).
According to Ginsburg et al., postponing surgery for up to 12 months fails to upgrade the disease (35); however, men with high-risk diseases who receive delayed treatment might have higher rates of biochemical recurrence (36-38). Although ADT before surgery is not recommended for patients with intermediate and high-risk diseases, some practitioners believe that upfront ADT might be an option if delayed treatment is expected. In contrast, EAU does not recommend using neoadjuvant ADT in order for postponing radical prostatectomy, and suggests performing long-term ADT + EBRT as an alternative treatment (10).
In the case of locally advanced disease, it is important to initiate the treatment within six weeks (6).
In many cases, cancer treatment during the COVID-19 pandemic requires the reconsideration of the risk/benefit ratio for the patients (11).
Postponing a life-saving cancer treatment is a difficult decision to make, and any decision in this regard must be justified based on conclusive evidence. Radiotherapy is one of the principal treatments for prostate cancer. Some radiotherapy treatment protocols can be modified during the pandemic. In this regard, the radiation oncology expert panel has published a guideline containing recommendations on managing patients with prostate cancer during the pandemic. To this end, they have developed and applied "the RADS framework" which stands for remote visits, avoidance, deferment, and shortening of radiotherapy (11).
4.6. Avoidance of Radiation Therapy
Prostate cancer, in general, is a slowly progressive disease for which the benefits of routine localized treatments should not be overestimated. The low-risk prostate cancer is defined as: cT1-2a and GS < 7 and PSA < 10 ng/mL. Several trials have demonstrated that watchful waiting and active surveillance are reasonable treatment options for dealing with very low and low-risk prostate cancers as they have favorable outcomes (11, 12). Therefore, watchful waiting seems to be the most appropriate therapeutic option for these patients, especially for those aged over 75 years (7, 12, 13).
4.7. Deferral of Radiation Therapy
In favorable intermediate-risk prostate cancer (cT2b-T2c; PSA 10 - 20 ng/mL; grade group 1 - 2), active surveillance can be considered a feasible option since several studies with more than ten years of follow-up have confirmed its safety and efficacy (13). In the favorable intermediate-risk group, even active surveillance may be deferred for patients with Gleason 3 + 4 disease for 3 - 4 months (12). With androgen deprivation therapy (ADT), radiotherapy can be further deferred if deemed necessary (11, 12). If ADT cannot be given for any reason, it seems reasonable to offer immediate treatment to high-risk patients (PSA doubling times ≤ 3 months), with the consideration of possible morbidity and mortality resulting from COVID-19 exposure (11).
There is controversy over the initiation of post-prostatectomy radiotherapy. While some guidelines suggest early radiotherapy as the preferred option (11), others recommend that physicians should prefer salvage radiotherapy over adjuvant radiation therapy (12). However, both options seem reasonable provided that the situation of the pandemic is considered.
4.8. Shortening of Radiation Therapy
If the treatment is deemed necessary, some guidelines recommend designing the shortest possible fractionation schedule for the patients. One such schedule is stereotactic body radiation therapy (SBRT) in 5- to 7-fractions localized prostate cancer. Since this method of treatment cannot be implemented in the majority of our centers, it is not regarded as a standard method. One possible, practical alternative is 60 to 62 Gy in 20 fractions. For patients undergoing prostatectomy, a moderate hypofractionated regimen is preferred (e.g. 52.5 Gy in 20 fractions) (11).
4.9. Active Treatment
Patients with unfavorable intermediate and high-risk prostate cancers should receive active treatment (7, 13). A large retrospective study on more than 63800 patients with unfavorable intermediate, high- and very high-risk prostate cancer who had been treated with radiotherapy and ADT showed that later radiation initiation up to six months after ADT initiation was not associated with worse overall survival compared with radiotherapy initiation before ADT (39). Therefore, three to six months of neoadjuvant ADT followed by delayed radiotherapy (6 - 12 months later) could be a reasonable alternative to surgery in these patients (13).
International guidelines recommend that the shortest safe radiotherapy regimen should be offered to these patients. In contrast to current guidelines, ultra-hypofractionated regimens (5- to 7-fractions with a single dose ≥ 5 Gy) should not be considered standard for our patients, given its unfeasible implementation in the majority of our centers and the lack of robust data on the use of these protocols in high-risk patients (7).
4.10. Brachytherapy
Brachytherapy is not recommended during the pandemic's peak given its reliance on anesthesia. Besides, as the majority of centers are not capable of performing brachytherapy, this method cannot be considered an option during the pandemic (5, 6).
4.11. Unnecessary Procedures
Some procedures that do not impact on overall survival rates might be omitted during the pandemic (11). Fiducial markers and rectal spacers, for instance, can be omitted as they require either prolonged or repeated patient visits (5, 6, 29).
4.12. Palliative Radiotherapy
Hypofractionated protocols are recommended for the palliation of bone metastasis due to their similar efficacy and reduced number of visits compared to traditional methods (7). Therefore, 8 Gy in one fraction and 20 Gy in 5 fractions seem to be reasonable and feasible options.
4.13. Systemic Therapy
Patients with metastatic prostate cancer are often frail and have multiple comorbidities; therefore, the advantages/risks of systemic therapy need to be assessed (29). On the other hand, reducing visits to the clinic is essential in order to minimize exposure to COVID-19 infection; therefore, palliative treatments for symptomatic patients require in-depth discussion (40).
androgen deprivation therapy can be suspended for patients who have nonmetastatic prostate cancer and PSA doubling time of more than 9 months (41). For patients with sensitive metastatic prostate cancer, ADT + androgen receptor axis targeted therapy (ARAT) is the standard care during the covid pandemic (22, 42).
Considering the risk of neutropenia and subsequent hospital visits, some experts argue that it might be prudent not to prescribe docetaxel for the patients during the pandemic (22, 23, 42). In these cases, abiraterone acetate can be considered (22, 42). For patients whose LHRH agonist has started, the longest cycle should be used frequently (e.g., every 3 - 6 months instead of monthly injections) (43, 44).
According to EAU guidelines, for patients with castration-resistant prostate cancer (CRPC), treatments should be administered in less than 6 weeks (42); however, it is recommended that chemotherapy should be avoided as much as possible (42). When necessary, docetaxel should be offered with systemic G-CSF to minimize frequent visits to the clinic (43, 44).
According to the Canadian guideline, it is reasonable to offer ARATs as the first line option for patients with castration-resistant metastatic prostate cancer; if ARATs have been prescribed previously, however, docetaxel can be considered as the next option (23, 42). In these cases, the results of a detailed discussion and shared decision should be considered by the patient and healthcare providers (23).
For patients with bone metastatic CRPC alone, radium-223 may be prescribed instead of chemotherapy (23, 42). It has been shown that ADT might provide the patients with partial protection against COVID. As a result, patients who receive ADT might face a lower risk of COVID-19 infection (44).
This study faced some limitations. First, biases – cognitive bias, in particular – may have affected our study results; therefore, experts in both surgical and nonsurgical disciplines were included in the research team, and an expert team of epidemiologists was requested to check the quality of the studies in order to minimize the given biases. Second, the review stage was not flawless and, for instance, the incomplete retrieval of the identified studies on COVID-19 extensively published during the pandemic as well as the biased reports of the cases due to short follow up may have affected our study findings.
5. Conclusions
It was recommended that prostate cancer screening should not be performed in asymptomatic men during infectious disease pandemics like COVID-19. It was also suggested that the treatment modalities should be performed in the shortest possible time and in the safest way in order to deal with the given cancer.