1. Background
Breast cancer is a common disease in Iran comprising 24.1% of all types of cancer (1), and globally with over 2.2 million cases diagnosed annually (2). Breast cancer treatment decision-making is challenging, requiring full participation from both the patient and their healthcare team (3). Healthcare decision-making relies on respect for patient autonomy and mutuality between providers and care recipients (4). Given advances in medical technology and treatment options, there is a growing global emphasis on shared healthcare decision-making (5). Shared decision-making (SDM) in medical treatments reflects individualized choices made using the best evidence with clear information regarding treatment advantages and disadvantages when considering patients' preferences and values (6). In Iran, it is common for patients to not participate in their treatment decisions allowing the healthcare team led by the attending physician to conduct this essential role. Further, patients also trust their healthcare providers to make health decisions and accept the treatment provided without question (3).
Shared decision-making may contribute to improved patient self-efficacy, which is characterized by the patients' perceived confidence in their capacity to exert behaviors that result in desired outcomes (7). Patients with higher self-efficacy report lowered stress and better adaptation to cancer and the prescribed treatment (8). Self-efficacy in patients with breast cancer has also been associated with fewer cancer symptoms, improved self-image, and positive relationships with healthcare providers (9). Self-efficacy may also be translated into patients feeling more comfortable asking questions and expressing concerns, which can also be associated with improved relationships between health providers and patients (10). However, although patients with cancer desire information about their disease and treatment, they may lack clarity regarding appropriate questions. Likewise, healthcare providers may not be aware of the patient’s information needs while also being concerned about the consequences of information provision on the patient’s capacity to cope (11).
In high-stakes situations such as determining the best option for cancer treatment, decision-making is considered high-quality when it is based both on the latest scientific evidence and also in accordance with the patient's values and beliefs associated with the potential outcomes (12). To determine patients' values and preferences in this regard, they must be involved in the decision-making process regarding treatment planning.
Patient decision aids, such as question prompt lists (QPL) have been developed to facilitate communication, enhance patient-centered care, and support SDM (13). The QPL is one of the simplest decision aids, consisting of an organized list of questions that patients can ask their health provider. The QPL guides patients in obtaining health information that is tailored to their unique needs and circumstances (10). Thus, the QPL can support patients in making informed decisions based on their values and preferences by providing questions that patients can rely on for obtaining information tailored to their educational needs. These questions are broadly generated and include impacts on quality of life and function in addition to impacts on survival and other associated parameters.
There have been mixed findings on the effects of QPLs on decision-making outcomes including treatment planning. For example, some studies have reported that QPL had no effects on whether patients asked questions about proposed treatments (14-16), whereas other studies have reported that utilizing QPLs can increase patients' capacity to ask questions (17, 18). Other studies assessing the effects of QPLs on the number of questions asked (14, 19), question content (17, 20), the information provided (21), knowledge recall (22), presence of anxiety (22), patient satisfaction (15, 22), and length of consultations (20, 22) have been evaluated with variant findings reported. Few studies have tested the effectiveness of QPLs on SDM in patients with cancer (23). The purpose of the current study was to investigate the impact of using a QPL among Iranian women with breast cancer following surgery on various decision-making outcomes, including SDM, decision-making self-efficacy, and preference for participation in decision-making.
The study is based on the self-efficacy theory originally developed by Bandura (24). The theory articulates that human belief in their personal agency to impact desired goals is essential for attaining desired outcomes. Self-efficacy can be impacted by cultural beliefs and norms and varies in accordance with differing situations (24). The decision-making capacity of patients in healthcare settings is directly related to their perceived self-efficacy (25).
2. Objectives
We hypothesized that providing a QPL to women facing treatment following surgery for breast cancer would enhance their perceived capacity to participate in their treatment planning and decision-making process.
3. Methods
3.1. Study Design
A randomized controlled trial was conducted, using 2 parallel arms. To minimize the possibility of confounding factors between groups, patients were randomized to receive either the QPL combined with usual care or usual care only.
3.2. Study Setting, Sampling, and Participants
The sample included post-surgical patients with breast cancer referred for follow-up treatment planning at a large comprehensive cancer center in Tehran, Iran. Based on their unique circumstances, the patients were to make decisions regarding follow-up chemotherapy, radiotherapy, hormonal treatments, multi-modality treatments, or no follow-up treatment. Inclusion criteria were: (1) patients with breast cancer who were referred for treatment planning following tumor resection; (2) knowledge about the diagnosis of breast cancer; (3) ability to read and write in Persian; and (4) age 18 years or older. Exclusion criteria consisted of (1) a history of psychiatric illness or cognitive disabilities that would impede the capacity to participate in treatment decision-making; (2) diagnosis of metastatic disease status post-surgery; and (3) reluctance to participate in research.
Using consecutive sampling, participant recruitment occurred from September 2019 to November 2020 to attain a sample size of 50 with 25 participants in each study condition. This sample size was estimated, using OpenEpi software based on the differences between the 2 means for the primary outcome (SDM). Specifically, considering a change of 5 scores as a criterion for the effectiveness of the intervention (power = 80%, α = 0.05) using means and standard deviations derived from a previous study (6), the necessary sample size was estimated at 22 per group. With predicted attrition set at 10%, 25 patients were enrolled for each condition.
3.3. Intervention
As summarized in Figures 1 and 2, the current study tested a QPL, consisting of an organized list of 14 questions for each treatment in 3 domains: (1) information about the malignancy; (2) treatment options; and (3) choices for follow-up after treatment. To develop the QPL items and to ensure their comprehensiveness, 3 sources of information were utilized: (1) existing evidence from other chemotherapy and radiotherapy-related QPL (22, 26-28); (2) expertise from the healthcare team in the radiotherapy-oncology department; and (3) interviews conducted with patients before the current study.
These patients identified various problems that they experienced and provided information about the questions they would have desired to ask but did not. The findings from these 3 data sources were integrated to form the final QPL. Before study initiation, this finalized QPL was tested for validity by several experts at the Tehran School of Nursing and Midwifery at Tehran University of Medical Sciences and experts at the Cancer Institute, Imam Khomeini Hospital to ensure accuracy and comprehensiveness.
3.4. Procedures
Following the completion of informed consent and baseline surveys, participants were randomized into either the usual care or experimental condition. For the randomization, allocation involved random sequence generation, allocation concealment, and implementation of the random allocation process (29). The production of a random sequence was carried out by the permuted block randomization method, using the web (30) with a fixed block size of four. Allocation concealment was carried out, using sealed opaque envelopes to have a non-predictable sampling. A research assistant performed these two steps, and the researcher who did the sampling did not participate in these two steps.
Patients who were randomized to usual care received information about their respective treatments. The treatment group received QPLs based on the potential treatments (i.e., chemotherapy, radiotherapy, a combination of both) in addition to usual care. Patients in this group were asked to mark any relevant questions in the QPL to ensure recall, and they received a follow-up phone call to remind them to use the QPL. Answers to the QPL questions were, then, provided in subsequent consultation in the form of face-to-face contacts, telephone, or through the WhatsApp social network tool. Patients were also able to ask questions that were not included in the QPL. Although few in number, answers to such questions that came up outside the patient visit were communicated to the researcher before the oncologist consultation and were discussed with the patients after coordination with the physician. Patients were informed that they were free to continue asking subsequent questions until their final treatment decision. The duration of utilizing the QPLs varied among patients and was quantified as a study variable by tracking days until a decision for specific treatments was made. Post-surveys were completed by all participants following the decision-making process for the respective medical treatments (measures are described next).
3.5. Measures
3.5.1. Demographic and Health Information
Demographic and health information included age, level of education (less than high school, high school diploma, and college degree), treatment type (chemotherapy, radiotherapy, and combination), history of chronic illness, and health information sources (media, family/friends, and healthcare team.
3.5.2. Shared Decision-making
Shared decision-making was assessed, using the 9-item Shared Decision-making Questionnaire (SDM-Q-9) (31). Response options are rated on a 6-point Likert scale (completely disagree = 0 to completely agree = 5) with scores ranging from 0 to 45. The previous reliability of this questionnaire in the study of Kriston et al. reported a Cronbach's alpha (α) = 0.938 (31). In this study, Cronbach's alpha was calculated at 0.7.
3.5.3. Control Preferences
Control preferences were assessed, using a descriptive statement-sorting version of the Control Preferences Scale (CPS) (32). The CPS has been utilized in cross-cultural studies globally, including Sweden (33), United Kingdom (34), Germany (35), Italy (36), and Norway (37-39). The CPS evaluates the preferred role of patients in participating in decision-making, with 5 options ranging from "A = independence in decision-making" to "E = complete authority of the physician in decision-making". Patients were first asked to choose an option based on the role they prefer in the decision-making process. Then, this action was repeated once more. Based on the preference, response options are classified as follows: A and B as active roles, C as a shared role, and D and E are inactive. If there was no consistency in the two actions in the response option classifications (e.g., A (active) after C (inactive)), the test was repeated. Hence, 6 scores were possible: Active-active (1); active-shared (2); shared-active (3); shared-inactive (4); inactive-shared (5); and inactive-inactive (6). These scores were, then, reported as 3 groups: Active (active-active or active-shared); shared (shared-active or shared-inactive), and inactive (inactive-shared or inactive-inactive) (6). The reliability of this scale was measured in the study of De Las Cuevas and Penate (Cronbach's α = 0.72) (40). In this study, Cronbach's alpha was calculated at 0.9.
3.5.4. Decision Self-efficacy
Patients’ confidence in making informed decisions about treatment was measured, using the decision self-efficacy (DSE) Scale developed by O’Connor. The decision self-efficacy questionnaire consists of 11 questions, in which the patient scores each question from zero (lack of self-confidence) to 4 (high self-confidence). The sum of the scores is divided by 11 and, then, multiplied by 25. Accordingly, scores range from zero (low self-esteem) to 100 (high self-esteem) (41). The reliability of this scale was measured in the study of Bunn and O'Connor (Cronbach's α = 0.84) (42). In this study, Cronbach's alpha was calculated at 0.8.
The face and content validity of the above-mentioned instruments were assessed and confirmed by 11 faculty members of School of Nursing and Midwifery of Tehran University of Medical Sciences.
3.6. Blinding
This study was single-blinded, such that, the statistician was not aware of the allocation of patients, whereas patients were provided with information as to their treatment arm.
3.7. Data Analysis
IBM SPSS 26 software was used for data analysis. The main analysis was performed according to an intention-to-treat approach. Descriptive statistics included frequencies, percentages, means, and standard deviations. The Shapiro-Wilk test was used to determine the normality of the data for the SDM-Q-9 and DSE scales. Fisher's exact test and independent t-test were used to compare demographic and health information characteristics and CPS scores between the two study conditions. Mann-Whitney U test was used to analyze scores of SDM-Q-9 and DSE scale for both groups in the pre-and post-tests. The significance level was set at P < 0.05.
3.8. Ethics
The clinical study was approved by the Ethics Committee of Tehran University of Medical Sciences and registered in the Iranian Registry of Clinical Trials (IRCT20190626044032N1). A signed informed consent form was obtained from all participants before study participation. Eligible patients were informed that their treatment would not be affected if they opted not to participate. Anonymity for those participating was assured, and all study materials were assigned codes as opposed to personal identifiers.
4. Results
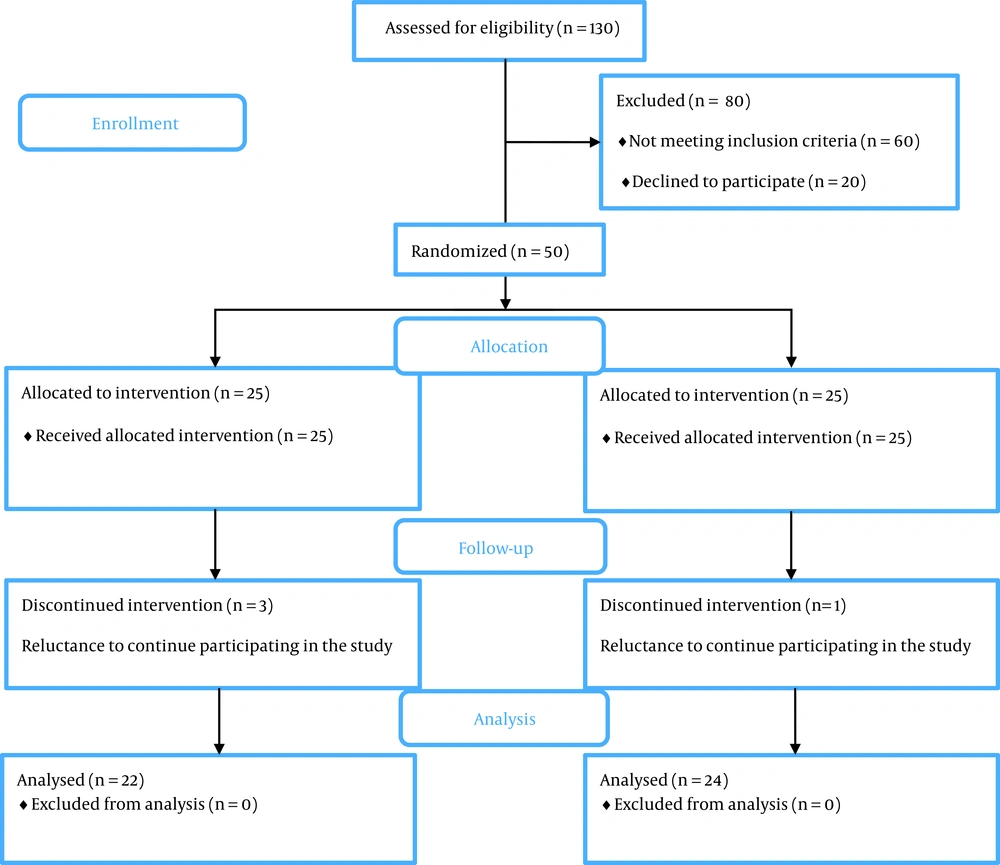
There were 25 patients enrolled in both the usual care and intervention conditions. Four participants (n = 3 in the control; n = 1 in the intervention) withdrew from the study before study completion. The study consolidated standards of reporting trials (CONSORT) flow diagram is provided in Figure 3.
Table 1 reveals the demographic and health characteristics of the study participants. Participants in the intervention and control groups were similar in terms of age, education, and history of chronic illness. In terms of follow-up treatment, more participants in the intervention group had radiotherapy only (n = 24, 96%) as opposed to the control group (n = 21, 84%). The median and interquartile range (IQR) were used to provide a comparison of SDM and decision self-efficacy across the study conditions, while frequencies and percentages were used to compare participation preferences. Median (IQR) scores on the SDM-Q-9 were 43 (5.75) for the intervention group and 40 (7.5) for the control group. Pre-test median (IQR) scores on the DSE were 93.18 (11.36) for the intervention group and 95.45 (9.66) for the control group. Post-test mean scores on the DSE were 97.72 (9.09) for the intervention group and 97.72 (7.39) for the control group. The results of pre-test comparisons indicated homogeneity of the two groups at baseline. Furthermore, a comparison of the primary outcome (SDM) and secondary outcomes (DSE and CPS) in post-tests revealed that there were no significant differences between groups (P > 0.05). Mann-Whitney U test on pre- and post-test DSE score differences revealed that there were no significant effects of participating in the intervention on decision self-efficacy (P > 0.05). Table 2 provides a comparison of decision-making outcomes by groups.
| Total Sample (n = 50) | Intervention Group (n = 25) | Control Group (n = 25) | |
|---|---|---|---|
| Age (y) | 50.04 ± 11.36 | 51.71 ± 13.10 | 48 ± 9.49 |
| Kind of treatment | |||
| Chemotherapy | 1 (2) | 0 (0) | 1 (4) |
| Radiotherapy | 45 (90) | 24 (96) | 21 (84) |
| Chemotherapy and radiotherapy | 4 (8) | 1 (4) | 3 (12) |
| Education | |||
| Less than high school | 28 (56) | 13 (52) | 15 (60) |
| High school diploma | 11 (22) | 6 (24) | 5 (20) |
| College degree | 11 (22) | 6 (24) | 5 (20) |
| History of chronic illness | |||
| No | 38 (76) | 18 (72) | 20 (80) |
| Yes | 12 (24) | 7 (28) | 5 (20) |
| Health information sources | |||
| Media | 7 (14) | 1 (4) | 6 (24) |
| Family and friends | 4 (8) | 3 (12) | 1 (4) |
| Health team | 12 (24) | 5 (20) | 7 (28) |
| Combination of above | 27 (54) | 16 (64) | 11 (44) |
| Number of days before treatment decision made b | 15.93 ± 7.54 | 16.71 ± 8.52 | 15.09 ± 6.39 |
Demographic Characteristics and Health Information a
| Control Group Median (IQR) | Intervention Group Median (IQR) | Mann-Whitney U, P-Value (Z Score) | |
|---|---|---|---|
| DSE scale (pre-test) a | 95.45 (9.66) | 93.18 (11.36) | 271.00, P = 0.41, (-0.81) |
| DSE scale (post-test) b | 97.72 (7.39) | 97.72 (9.09) | 248.50, P = 0.72, (-0.35) |
| Pre-test and post-test DSE scores difference | 0.000 (7.39) | 2.27 (6.82) | 231.50, P = 0.47, (-0.72) |
| SDM-Q-9 b | 40 (7.5) | 43 (5.75) | 325.50, P = 0.17, (-1.37) |
| No. (%) | Fisher's Exact Test | ||
| CPS (pre-test) a | P = 1.00, df = 1 | ||
| Active | - | - | |
| Collaborative | 2 (8) | 1 (4) | |
| Passive | 23 (92) | 24 (96) | |
| CPS (post-test) b | P = 0.46, df = 2 | ||
| Active | 1 (4.5) | 1 (4.2) | |
| Collaborative | 2 (9.1) | 0 (0) | |
| Passive | 19 (86.4) | 23 (98.8) | |
Decision-making Outcomes by Group
5. Discussion
The study evaluated the role of a QPL in impacting decision-making outcomes in women facing adjuvant therapies following breast cancer tumor resection surgery. The findings of this study did not identify differences in decision-making parameters among women, who used the QPL compared to women who did not use QPL.
Previous research has found that the QPL can increase the perceived capacity of patients to ask questions (17, 18). Thus, we hypothesized that if the QPL could increase patients' ability to ask questions, it could also facilitate SDM. However, the findings of our study did not support this hypothesis. Instead, findings are consistent with other researchers, such as Henselmans et al. (43), who did not find that a patient communication aid (of which QPL was one of the components) affected SDM in patients with advanced cancer. Similarly, Amundsen et al. (44), reported that a combination of interventions including QPL and consultation audio recordings did not affect SDM in their quasi-experimental study that also included patients with cancer. Importantly, the results of our study were consistent with both previously mentioned studies, and the findings build on previous findings. For example, in the present study, only the effect of one intervention (QPL) was investigated, whereas, in the previously mentioned research, combination interventions were used. In the present study, SDM scores were similar in both the control and intervention groups.
The results showed that the QPL was not effective in enhancing decision-making self-efficacy in this sample of women with breast cancer. Reumkens et al. (45) examined the effect of a decision aid on self-efficacy in persons with a genetic predisposition to cancer and their partners during reproductive decision-making. They reported that the decision aid was able to improve decision self-efficacy in people, who initially had low self-efficacy. In contrast, in people who initially had high self-efficacy, scores decreased within 2 weeks of using a decision aid. In our study, decision self-efficacy scores were not affected by the QPL.
The results also found that the QPL did not impact preferences for participation in decision-making among this sample of women with breast cancer. Participation preferences can vary in different cultures. For example, a study in Jordan found that 50% of patients preferred a passive role in medical decisions, such that decisions are made on their behalf (46), whereas in a study conducted in the Netherlands, it was reported that 85% of lung cancer patients preferred a shared role in decision making (47). Another study done in Iran that also included a sample with breast cancer found that most patients preferred having a passive role in treatment decisions (6). Also, the nature and severity of the illness under inquiry are factors that may impact preferences for participation. Given cancer is both life-threatening and life-limiting, patients may delegate decision-making to their providers (6, 48). Such factors may play a role in the findings that were found in the current study. Further, it is important to recognize that the inclusion of a QPL is not a regular practice in Iranian culture. Women are not sensitized to consider that they have an opportunity to participate jointly in treatment decision-making in Iran and such practices may seem foreign. This may have impacted how the QPL was utilized by the treatment condition. With health cultural shifts towards patient-centered care, SDM may become more normalized and accepted. The current study was important as an early step in evaluating how the provision of active information and questions could potentially galvanize patients to sense that they had a voice in how treatment decisions were made.
There were several limitations in this study. The sample was constrained by a small sample size and a convenience sample of participants with breast cancer. Given participants were post-surgical, treatment factors associated with recent healthcare stressors could have impacted their perceptions and responses to survey items. Further, the last 6 months of the sampling phase coincided with the COVID-19 pandemic, which contributed to fewer patients and a prolongation of the study’s sampling phase. Future research should, thus, evaluate whether QPL targets SDM by encouraging more questions to be asked by patients.
5.1. Practice Implications
Nurses, as members of the health team, spend substantial time interacting with patients. Nurses can encourage patients to participate in treatment and care. Patient education that includes information about SDM and the importance of asking questions can be incorporated as a standard of practice. More research is needed on determining how decision aids such as the QPL can be adapted to better impact decision-making outcomes through supporting patient autonomy and personal preferences. Such studies are needed not only in cancer but also in relation to other healthcare issues and populations.
5.2. Conclusions
The study determined that the QPL did not impact various decision-making outcomes among women with breast cancer. Previous evidence (6) has suggested that the preference for participation in treatment decisions among women with breast cancer in the Iranian culture is more passive. Further studies are needed to identify reasons for patients being less included in participation in treatment decisions. Future research should evaluate the timing of when decision aids are introduced during the treatment planning process. As patient-centered care and SDM contribute to better outcomes, strategies must be evaluated to improve the capacity of Iranian women to participate actively in their care.