1. Introduction
Usually, the accompaniment of basal cell carcinoma (BCC) with meningioma has been seen with an association of syndromes such as neurofibromatosis or a previous history of radiotherapy which alleviate the expression of cancer-like lesions. This article reports a case of accompaniment of meningioma and basal cell carcinoma with no other symptoms of allied usual syndromes.
2. Case Presentation
A 62 years old man was admitted to the outpatient neurosurgical clinic with the chief complaint of left inferior limb weakness. He also mentioned occasional monoparesis of a left inferior limb which resolved spontaneously, non-specific headaches, and somnolence. The weakness had happened 2 or 3 more times before and resolved without any special treatment. We think these weaknesses could be a new onset seizure.
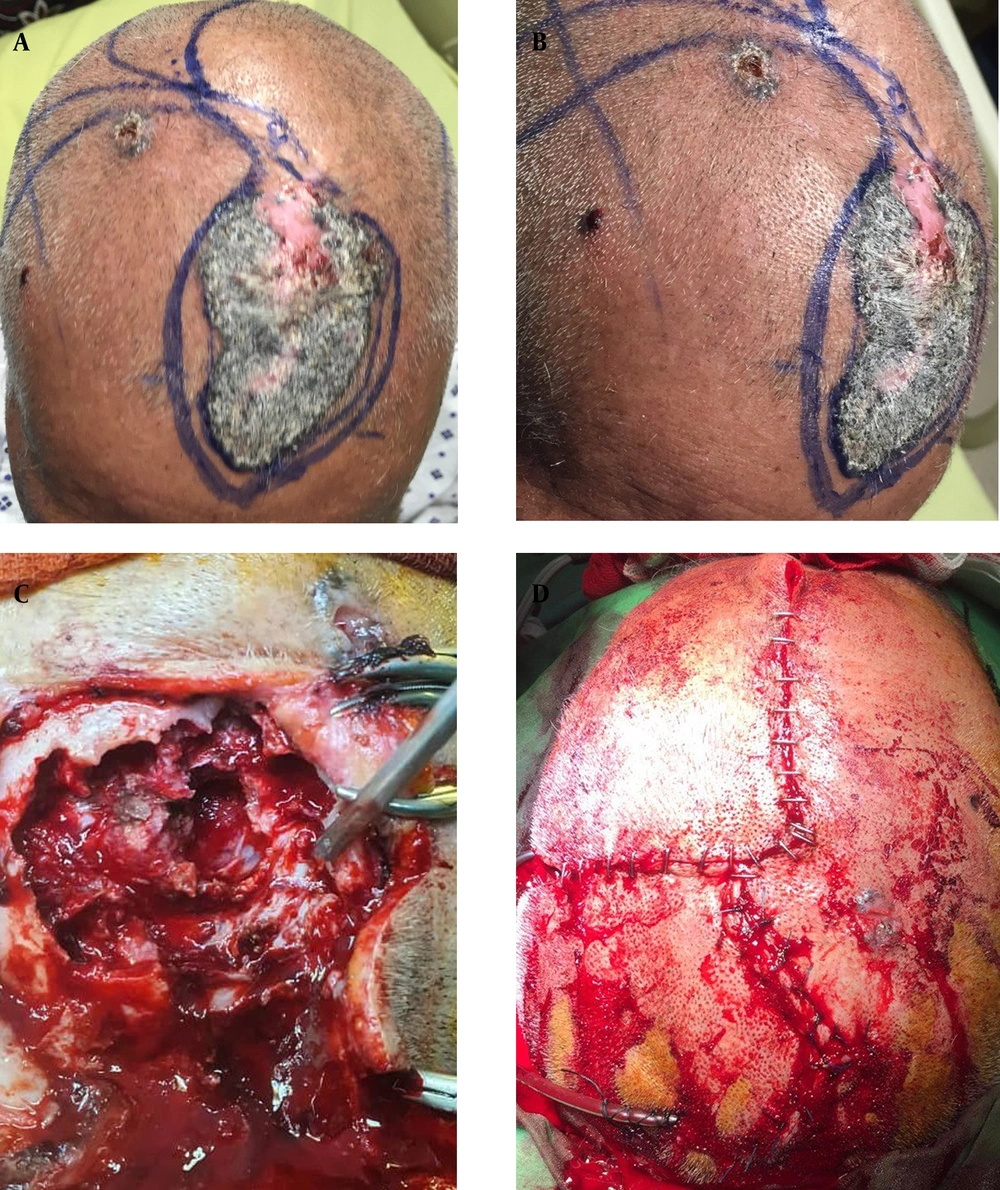
In objectives, there was a huge skin lesion on the frontoparietal lobe of the skull with a diameter of 10 × 7 cm (Figure 1A - D). A prominent subcutaneous lesion with a vertical diameter of 0.5 cm and horizontal diameter of 1 cm. Also, there were 8 more small lesions all over the body. There were no cafe au lait lesions over the body surface.
For neurological symptoms, considering the chief complaint we did a physical examination. A neurologic physical exam revealed hyperreflexia and parietal lobe syndrome in the right lobe: Tactile and visual extinction and neglect of the left side. Decrease in left limbs force: Lower limb: 3/5, upper limb: 4/5, and hemiparesis of the left side with the preference of lower limb.
Ocular examination showed papilledema and visual field defects. In the skin exam, there was a wide lesion with a diameter of 10 × 7 cm on the frontoparietal region of the scalp, in the accompaniment of 8 more lesions on the scalp and all over the body.
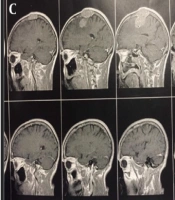
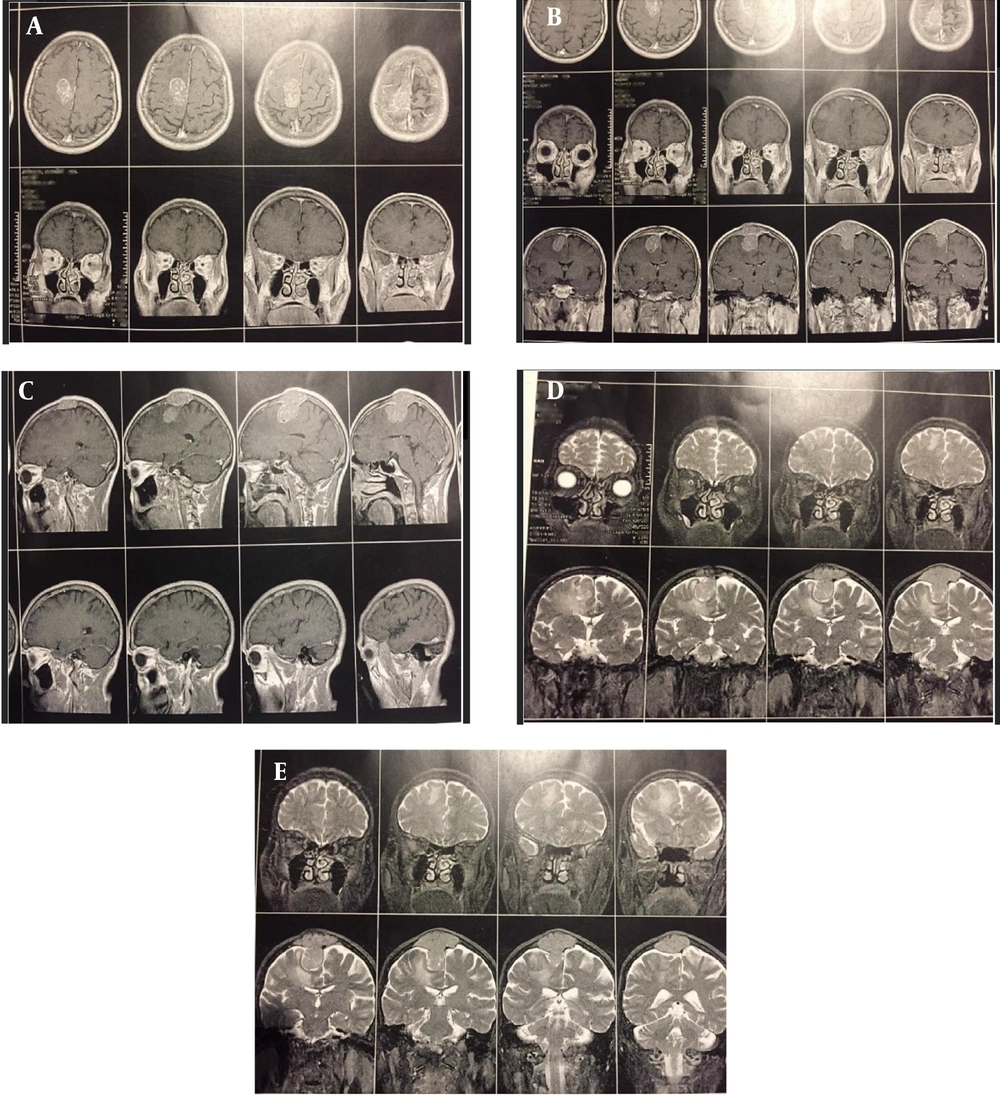
We performed brain-MRI with and without GAD (Figure 2A - E). A popcorn-shaped, homogenous, well-defined, En-plaque parasagittal mass with high vascularization, hard consistency, and compressive effect on the parietal cortex with greater exophytic part and invasion to the bone with a diameter of 4 cm and skin was seen.
No familial history and no previous irradiation were found.
Informed consent was obtained from the patient for further studies and reports on this case.
2.1. Surgical Methods
An S-shaped incision was performed with 4.5 cm space from the anterior scalp lesion to prevent misalignment and disintegration of skin after plastic surgery. A craniotomy with a margin of 1 cm from the bone invasion of the mass was performed. A cross-shaped durotomy was performed. After inspection of the intact cerebrum, gross total resection of the tumor and arachnoid plane were performed. In the end, cranioplasty with an intact bone margin of 1 cm was performed.
The tumor was sent to the pathology lab and reported an En-plaque parasagittal fibroblastic meningioma (Figure 3).
Later in the same operation, the dermatology team performed a skin biopsy from the anterior scalp lesion and other lesions. Skin biopsies were sent to the pathology lab. All skin lesions reported by pathology lab as meta-typical basal cell carcinoma.
3. Discussion
There are no previous recordings of accompaniment of parasagittal En-plaque fibroblastic meningioma and basal cell carcinoma which is not Gorlin-Goltz syndrome. This patient’s skin lesions were not neurofibromatosis-like. The patient did not have a history of radiotherapy.
Reading about this case might bring cutaneous meningioma to mind but the pathology reported BCC and En-plaque meningioma separately, which is the unique aspect of this case. In this case, the relationship between dermatological cancer and brain cancer was grotesque.
It is known that the BAP-1 gene is predisposing to BCC, meningioma, melanoma, renal cell carcinoma, etc (1). Although in this patient we did not observe any Gorlin-Goltz syndrome symptoms. It is known that meningioma can induce neoplasm in its environment due to its impact on cytokines and cell division. Mostly, astrocytoma or glioma is reported before (2).
In 2007, Bajetto et al. showed the expression of human stromal cell-derived factor-1 (SDF-1) and its receptor CXCR4 in human meningiomas (3). Previously they have reported the expression of these chemokines in human gliomas. It is known that SDF-1 induces mitogenic activity via upregulation of its receptor CXCR4 (4-6).
It has been reported that meningeal cells synthesize and secrete SDF1 and its receptor CXCR4 (7, 8). It can attract stem cells to increase proliferation. Neural cells and also meningioma tumor cells express CXCR4 and SDF-1 (4). It has been reported in 2015 by Quan, et al. that normal skin cells express SDF1 and CXCR4 (9). Furthermore, many different cancer cells up-regulate the expression of the mentioned factors too, such as basal cell carcinoma, squamous cell carcinoma, and psoriasis (9).
We hypothesize that as the meningeal cell expresses SDF-1 and CXCR4 to absorb the stem cells it might cause the aggregation of stem cells and produce a meningioma mass. In addition, somehow it may cause the up-regulation of CXCR4 and SDF-1 in skin cells and result in skin cancer, and in this case basal cell carcinoma. This is a raw hypothesis and it needs experiments to be conducted to elucidate the process of causation. The neoplasm-inducing effect of meningioma has been seen in cases with an accompaniment of glioma too.
It is reported in 2013 by Bollag and Hill that the CXCR4 and SDF-1 are seen in keratinocyte proliferation in psoriasis, basal cell carcinoma, systemic lupus erythematosus, and skin wound healing (10).
We think these cases of accompaniment of meningioma with skin cancers or glioma, are good examples of meningioma local neoplasm-inducing features. More studies are needed to answer the further questions every case brings to mind.