1. Background
Gastric cancers (GCs) represent one of the most common reasons of cancer death worldwide, and it is now the fourth most common malignancy leading to high morbidity and mortality (1). The median age of most patients is about 70 years and about 10% are 45 (2). The regions with an increased incidence of GC are Asia, Central and South America, and Eastern Europe, with notable low survival despite the remarkable progress in the diagnosis and early prediction of the pathophysiological behavior of cancer as well as effective treatments (2, 3). Comprehensive risk factors for GC have been identified, which include a set of individual characteristics, lifestyle, genetic, and environmental factors. In this regard, demographic parameters, ethnicity, infection with Helicobacter pylori, smoking, and high-nitrates dietary regimens have been identified as the potential risk profiles for GC (4-6). The role of genes and the factors coded by them in the occurrence and progression of GC has been thoroughly proven. A network of interactions between genes, transcription factors, signaling pathways and protein enzymes act in the proliferation, and continuation of the cycle of tumoral cells (7). So, some biomarkers have been described for the diagnosis and outcome prediction of cancers (8). Any alteration of the promoter or suppressor oncogenes encoding regulators of cell cycles contributes to carcinogenesis and invasive behaviors of cancer, such as invasion and metastasis. One of the main regulatory factors of tumor cell cycles is the P21 protein. P21 is an inhibitor of the cyclin-dependent kinase, which its coding gene locates on chromosome 6. P21 protein attaches to the cyclin-dependent kinase (CDK) and inhibits its activity, thus playing a role as a cell cycle regulator in G1 and S phases (9). Loss of the cell cycle regulator gene is an important happen in tumor progression (10). Also, by inducing the activity of P53, the P21 protein can prevent the proliferation of cancer cells with DNA damage (11). Studies have shown that malignant cells with a high level of P21 were quiescent in the G0 state, while cells with low levels of P21 continued to increase. Thus, the suppressing role of P21 on cancer cell proliferation has been accepted (12). The role of the P21 in GCs has been noticed; however, its expression and diagnostic importance in GCs and its association with histopathologic characteristics of the tumor have not been correctly determined.
2. Objectives
In this research, our goal was to evaluate the expression level of P21 in GCs and to recognize its association with the histopathological findings of cancer.
3. Methods
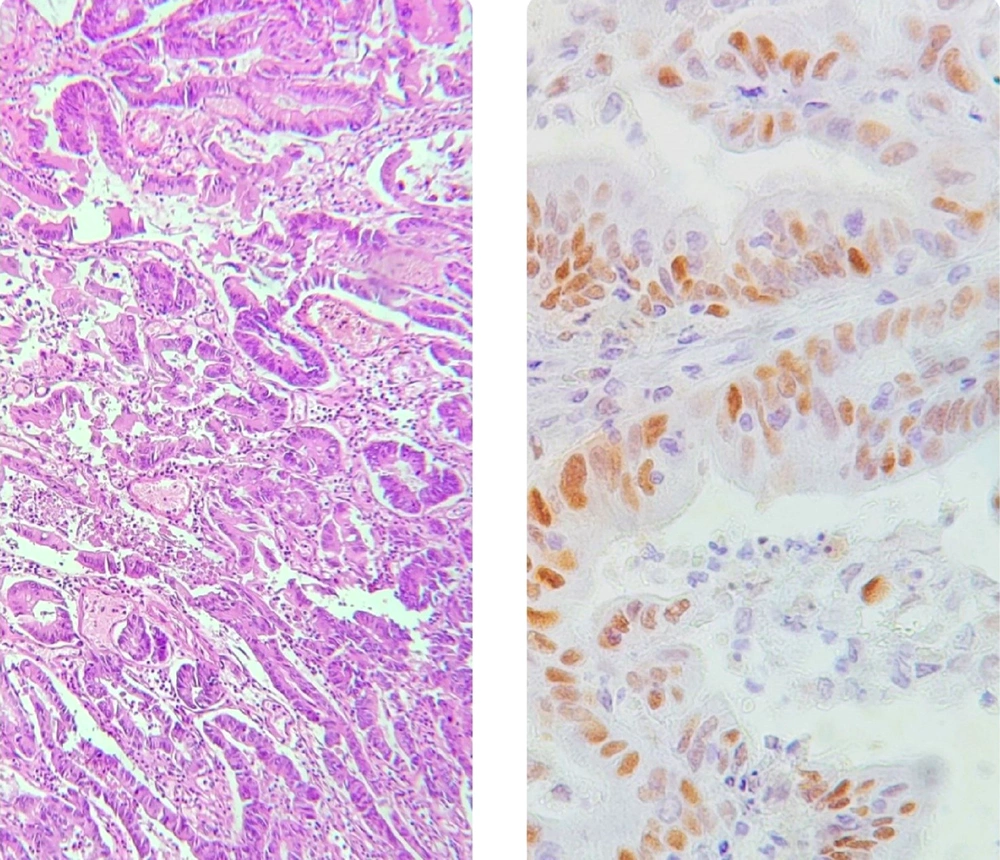
Our study was cross-sectional and performed on patients with GCs who underwent radical gastrectomy at Sina hospital in Tehran between 2021 and 2022. The pathological diagnosis was according to the College of American Pathologists (CAP) classification. Therefore, those who did not schedule for radical gastrectomy or the pathological classification of lesions not according to the CAP criteria were not included in our study. The baseline characteristics, including demographics, were collected by reviewing the hospital files. Paraffin block from neoplastic tissue on gastrectomy specimen was prepared. For pathological evaluation, the samples were sent to the hospital’s laboratory. They assessed the type of gastric adenocarcinoma (diffuse (signet) or intestinal), tumor grade, tumor size and extension, and the existence of lymphovascular and perineural invasion. The histopathological evaluation was assessed by Hematoxylin & Eosin (H&E). The immunohistochemistry (IHC) technique was used to assess the expression of the P21 marker. Nuclear staining was considered positive. The study endpoint was to assess the relationship between the P21 positivity and histopathological characteristics of the tumor (Figure 1).
3.1. Statistical Analysis
The results for quantitative variables were as mean ± standard deviation (SD) and for categorical variables, were by rate (percentage). The Mann-Whitney U test or t-test was used for continuous variables when data did not display to have a normal distribution. The chi-square test was used for the comparison of the categorical variables. P-values less than 0.05 were designated statistically significant. The statistical software SPSS version 23.0 for windows (IBM, Armonk, New York) was used for the statistical analysis.
4. Results
Overall, 40 patients suffering from gastric adenocarcinoma were included in our study. The median age of the GC patients was 63.20 ± 10.72 years (between 39 to 84 years), and 75.0% were male. Regarding pathological indices of the tumor, 82.5% of the lesions were intestinal type, and others were diffuse (signet) type. In total, 32.5% were graded as I, 27.5% were graded as II, and 40.0% were graded as III. The lesions were mostly located in the gastric antrum (50.0%) and cardia (27.5%). Lymphovascular and perineural invasions were revealed in 45.0% and 42.5%, respectively. More than half of the lesions were categorized as stage III (57.5%). The mean size of the lesions was 4.30 ± 2.61 cm, ranged 1.5 to 10 cm. The demographic data and tumor-related parameters are shown in Table 1.
| Variables | Values |
|---|---|
| Average age, y | 63.20 ± 10.72 |
| Male gender | 30 (75.0) |
| Tumor type | |
| Intestinal | 33 (82.5) |
| Diffuse (signet) | 7 (17.5) |
| Tumor grade | |
| I | 13 (32.5) |
| II | 11 (27.5) |
| III | 16 (40.0) |
| Location | |
| Antrum | 20 (50.0) |
| Body | 7 (17.5) |
| Cardia | 11 (27.5) |
| Fundus | 2 (5.0) |
| Perineural invasion | 17 (42.5) |
| Lymphovascular invasion | 18 (45.0) |
| Tumor stage | |
| I | 5 (12.5) |
| II | 6 (15.0) |
| III | 23 (57.5) |
| IV | 6 (15.0) |
| Mean size, cm | 4.31 ± 2.61 |
| P21 positivity | 28 (70.0) |
a Values are expressed as mean ± SD or No. (%).
In total, P21 positivity was revealed in 28 specimens of the lesions, with an overall prevalence of 70.0%. As indicated in Table 2, the positivity for the P21 marker was independent of patients’ age and gender. There was no association of the P21 positivity with tumor-related characteristics, including tumor location, type, grade, stage, as well as the size of the lesion. Similarly, P21 positivity could not be related to perineural or lymphovascular invasion of the tumor.
| Characteristics | Positive P21 | Negative P21 | P-Value |
|---|---|---|---|
| Average age, y | 62.29 ± 11.00 | 65.33 ± 10.14 | 0.417 |
| Gender | 0.693 | ||
| Male | 20 (71.4) | 10 (83.3) | |
| Female | 8 (28.6) | 2 (16.7) | |
| Tumor type | 0.410 | ||
| Intestinal | 24 (85.7) | 9 (75.0) | |
| Diffuse (signet) | 4 (14.3) | 3 (25.0) | |
| Tumor grade | 0.771 | ||
| I | 10 (35.7) | 3 (25.0) | |
| II | 7 (25.0) | 4 (33.3) | |
| III | 11 (39.3) | 5 (41.7) | |
| Location | 0.309 | ||
| Antrum | 15 (53.6) | 5 (41.7) | |
| Body | 3 (10.7) | 4 (33.3) | |
| Cardia | 8 (28.6) | 3 (25.0) | |
| Fundus | 2 (7.1) | 0 (0.0) | |
| Perineural invasion | 0.530 | ||
| Positive | 11 (39.3) | 6 (50.0) | |
| Negative | 17 (60.7) | 6 (50.0) | |
| Lymphovascular invasion | 0.071 | ||
| Positive | 10 (35.7) | 8 (66.7) | |
| Negative | 18 (64.3) | 4 (33.3) | |
| Tumor stage | 0.538 | ||
| I | 4 (14.2) | 1 (8.3) | |
| II | 5 (17.9) | 1 (8.3) | |
| III | 14 (50.0) | 9 (75.1) | |
| IV | 5 (17.9) | 1 (8.3) | |
| Mean size, cm | 3.85 ± 2.54 | 5.37 ± 2.56 | 0.091 |
a Values are expressed as mean ± SD or No. (%).
5. Discussion
Due to the lack of detailed imaging methods and the aggressive behavior of GCs, specific biomarkers for estimating the GC outcomes are needed (13). The progression of the cell cycle is regulated by different particular enzymes such as CDKs. These enzymes can phosphorylate some gene-coded proteins that progress the G1 phase into the S phase in the cell proliferation cycle. Thus, the inhibitors of CDKs can block its activation and may arrest the G1 to S phase and suppress the cell proliferation cycle. In tumor cells, this inhibition by CDKs suppressors may be considered as an alternative in suppressive tumor cell proliferation and invasion. P21 has been found to act as a CDKs suppressor, and thus, reducing P21 expression may be associated with tumor progression and lowering patients’ survival. Such a role for P21 has been reported by some authors in GCs (14-16). In other words, assessing the P21 expression in cancer cells can be prognostic and a useful marker of patients’ prognosis. However, it seems that such a genomic pattern can be affected by various factors, such as racial and environmental characteristics. Therefore, even in the case of high expression of this factor, such a prognostic role may not be observed in some societies.
As the first finding, we showed high expression and positivity of the P21 in gastric adenocarcinoma. P21-positivity in these patients was found at about 70.0%. Almost all similar studies could achieve high rates of P21 positivity in GC states. Ozen et al. study (17) showed that the P21 expression was positive in 61.4 % of GCs. In another study by Kouraklis et al. (18), the P21 expression was detected in 37.5% of GCs. In the study by Doganavsargil et al. (16), 31.3% of the neoplastic tissues exhibited any expression of P21. Ogawa et al. (14) also showed the P53 and P21 positive staining in 50% and 37.2% of tumors, respectively. In Seo et al. (15) survey, P21 and P53 expression in nuclear tumoral cells were proved in 63.7% and 33.3% of neoplastic specimens, respectively. Despite high P21 positivity in our patients, we could not show any association between P21 expression and the biological behaviors of cancerous cells. In other words, in our population, P21 positivity may not predict tumor progression, invasion or metastasis. However, several studies could demonstrate its prognostic role in gastric adenocarcinoma. In several studies, P21 negativity is associated with unfavorable outcomes in patients with cancer. As indicated by Ozen et al. (17), the lack of P21 expression was related meaningfully to diffuse and undifferentiated form histological features, complete involvement of the stomach and presence of lymphovascular or perineural invasion. The patients with no expression of P21 had a lower average survival rate than those with positive P21 expression. Kouraklis et al. (18) showed that positive P21 immunostaining was accompanied by less infiltration of tumoral cells to the gastric wall, a lack of lymphovascular infiltration as well as no tumor metastasis. Doganavsargil et al. (16) showed that P21 expression was more in men and patients with tumor extension in submucosal and atrophic gastritis. Seo et al. (15) also found no P21 expression related to an advanced stage and lymph node involvement. Lack of P21 expression is related to poor outcomes. Therefore, although P21 marker expression is considered an inhibitor of the cell cycle in cancerous cells, such a role was not observed in our study. This meaningless result can occur for various reasons. First, the expression of genes and markers coded by these genes can be completely depend on racial and geographical characteristics. Therefore, in Iranian society, this marker may not have a prognostic role. Secondly, the small sample size of the study as a potential limitation of this study had a substantial impression on the results. In order to attain reliable consequences, it will be necessary to evaluate the relationship between P21 marker expression and the biological and histopathological behavior of gastric tumors in the broader society, taking into account racial and geographic characteristics.
5.1. Conclusions
In conclusion, in our patients, P21 expression may not be linked to the inhibition of gastric cancer progression and invasion. In other words, P21 negativity may not indicate tumor progression and metastasis.