1. Context
Although the development of lung cancer treatment has experienced rapid progress, lung cancer still has high mortality and morbidity rates (1). One of the reasons lung cancer is at the top of this list is that it is often diagnosed at an advanced stage (2). Therefore, catching cancer in preneoplastic lesions that allow patients to be diagnosed at an early stage is critical (3). Currently, there are several modalities for the early detection of lung cancer, including sputum cytology examination, thoracic computed tomography (CT) scan, or X-ray. Furthermore, tissue biopsy examination through bronchoscopy procedures can be used as an early detection modality, thus increasing the likelihood of successful treatment (4).
Flexible bronchoscopy is a standard procedure recommended for patients with suspected lung cancer. Although it has fairly good diagnostic value, cancer lesions can be missed when examined with conventional bronchoscopy or white light bronchoscopy (WLB) alone commonly in the atypical malignant lesion (5, 6). The bronchoscopy imaging technology was developed 2 decades ago to improve test performance characteristics, especially narrow-band imaging (NBI), and to determine the exact pathologically altered airway and microvascular pattern of a biopsy. Narrow-band imaging with a high-resolution video imaging system can enhance image quality by enhancing the contrast of mucosa and submucosa vascularization. This feature may enable advanced diagnostic accuracy in detecting airway neoplasia lesions at a precancerous stage (6).
Several studies have explored the variable accuracy of NBI bronchoscopy for detecting airway cancer lesions. Previously, the NBI technique was successful in increasing the diagnostic yield and evaluating the vascularization on the surface lesion of the gastrointestinal system (7). Moreover, NBI allows the clinician to assess the pathologies of the head, neck, and throat inflammatory lesion (8). However, the role of the NBI bronchoscopy technique in the detection of premalignant airway lesions remains challenging. Several studies had reported the sensitivity and specificity at different thresholds.
2. Objectives
We conducted a systematic review and meta-analysis aimed at determining the diagnostic value of NBI bronchoscopy for diagnosing cancerous lesions of the airway. We also reviewed the risk of bias in each of the eligible studies.
3. Methods
This meta-analysis was prepared based on the preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA) and PRISMA 2020 guidelines (9). The search strategy, study selection, quality assessment, and data extraction were performed by 3 investigators. Quality assessment and data extraction were carried out independently by 3 investigators. Any differences were resolved by agreement among the 3 investigators.
3.1. Search Strategy
Three databases (PubMed, Scopus, and Embase) were used to conduct literature searches for papers. The Universitas Indonesia online library was used to access these databases, with the last search performed on 3 November 2022. As guidance for the literature searching and sorting of the clinical research question, we use the PICO model frameworks approach, where P (study population) = patients with suspected lung tumors or metastases; I (intervention) = NBI bronchoscopy imaging technique; C (comparison) = standard bronchoscopy, WLI bronchoscopy or without comparison; O (outcome) = diagnostic accuracy indicators, sensitivity, and specificity (10). The literature search was performed, using a combination of keywords based on the MeSH terms and Boolean operators (‘AND’ and ‘OR’) for each database. The words ‘narrow band image’ and similar terms, including ‘narrow band imaging’ and ‘narrow band bronchoscopy’ were connected by OR. The terms ‘lung cancer’, ‘lung tumor’, ‘lung metastatic’, and ‘lung malignancy’ were connected by OR.
The search was limited to human subjects and English-language studies, but not by age, date of publication, or country. We did not include literature from abstract meeting proceedings or unpublished studies due to the lack of detail and an in-depth review of these studies. The initial screening was based on the titles, including their abstracts of the retrieved documents, and continued by screening the full text, including available data extraction.
3.2. Study Selection
3.2.1. Types of Studies and Participants
Eligible studies investigated the diagnostic accuracy of bronchoscopy NBI for early detection of lung cancer, except for case reports, case series, reviews, editorials, and commentaries. Participants in each study were patients with suspected lung cancer, patients in a high-risk group for lung cancer, patients with a history of lung cancer or malignancy of other organs, and smokers.
3.2.2. Index Tests and Reference Tests
The index test that we used was the imaging of NBI bronchoscopy in a suspected patient at risk of lung cancer. The test was considered positive if there was at least one picture of vascular abnormalities on NBI bronchoscopy (11, 12). The reference test used was the histopathological results of carcinoma based on the World Health Organization’s (WHO) classification (13, 14). The reference test was considered positive if it was carcinoma and negative if it was non-carcinoma, including precancerous lesions.
3.2.3. Data Extraction and Quality Assessment Tool
These data were taken from each study: characteristics, participant characteristics, bronchoscopy machine and scope used, the number of operators, NBI bronchoscopy diagnostic criteria, and histopathological results. Quantitative data were obtained for constructing a standard 2 × 2 diagnostic test table. The extracted data were true positive (TP), true negative (TN), false positive (FP), and false negative (FN) from the eligible studies. Furthermore, the quality of each study was evaluated, using the quality assessment of diagnostic accuracy studies-2 (QUADAS-2) tool to evaluate the risk of bias and the quality of the diagnostic profile, which consists of these main domains: Patient selection, index test, flow, and timing of reference tests (15). The risk of bias and the applicability concern were divided and categorized as high, low, and unclear.
3.2.4. Statistical Analysis
We determined the diagnostic accuracy of NBI by calculating the specificity, sensitivity, positive likelihood ratio (LR +), and negative likelihood ratio (LR -) from each study with a 95% confidence interval (CI) of the NBI bronchoscopy procedure in diagnosing cancerous lesions in suspected lung tumor patients (16, 17). These diagnostic accuracy indicators were displayed in the forest plots. All statistical analyses were performed as a meta-analysis, using STATA. The studies’ heterogeneity was assessed, using the I2 test with an I2 value of ≥ 50% indicating significant heterogeneity (18, 19).
4. Results
4.1. Identification of Study
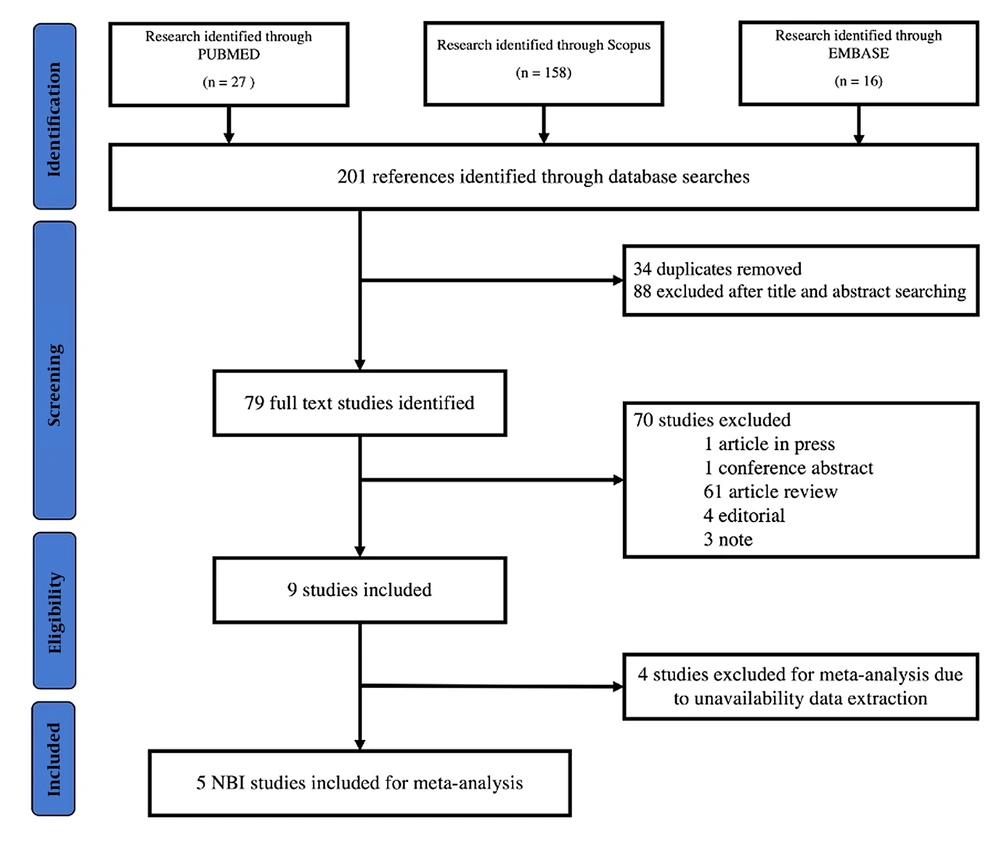
Our search strategy flow is shown in Figure 1. In the preliminary search, we identified 27 articles from PubMed, 158 articles from Scopus, and 16 articles from Embase. We excluded duplicate studies, articles without full text, and studies that did not comply with the eligibility criteria. Additionally, studies, where data could not be extracted, were excluded. Finally, we identified 5 articles eligible for meta-analysis and statistical analysis inclusion criteria.
4.2. Study Characteristics
Of the 5 included studies, 1267 patients underwent NBI bronchoscopy and 1850 biopsies were performed (20-24). The studies were conducted in the USA, China, Serbia, and India. The study characteristics and diagnostic accuracy of each study are detailed in Table 1. All studies were prospective and non-randomized studies, and one study was a pilot study. Two studies divided the subjects into two groups: NBI bronchoscopy and WLB. Three studies used flexible bronchoscopy with the Olympus system in the LUCERA series, one study used the EXERA II series, and one study was unclear about which series was used. The use of NBI bronchoscopy was performed after the WLB procedure. The criteria for defining the location of the biopsy were based on Shibuya’s criteria, which were determined by at least two bronchoscopists. One study did not mention the biopsy location. Two studies used oval cup forceps with spikes, one study used serrated jaw forceps, and one study was unclear about the biopsy method. The bronchoscopy technique, lesion characterization, and control for each included study are presented in Table 2.
| Author, Year | Country | No Patients (No. Male/Female) | No. Biopsies | Median Age (Min-Max) in Years | Study Design | Population | No. True-Positive | No. False-Positive | No. False-Negative | No. True- Negative |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Vincent, 2007 (24) | United States | 22 (8/14) | 64 | 59 (28 - 77) | Prospective, nonrandomized, compared to WL bronchoscopy | >18 years old, suspected airway cancer or dysplasia, abnormal sputum cytology examination radiology finding of airway stenosis from known or suspected lung metastases | 9 | 33 | 0 | 22 |
| 2. Bojan, 2009 (23) | Serbia | 36 (29/7) | 132 | 55 (25 - 79) | Prospective, nonrandomized | 18 years, thoracic X-ray or CT scan suspicious of lung malignancy, PS ECOG 0 - 2, without known suspected metastases from lung cancer, to assess tumor extension | 90 | 6 | 2 | 34 |
| 3. Advani, 2018 (21) | India | 187 | 102 | Not mentioned | Prospective, compared to WL bronchoscopy | > 18 years old history, signs, symptoms, and radiologic findings suggestive of lung cancer | 62 | 7 | 1 | 32 |
| 4. Zaric, 2009 (22) | Serbia | 106 (85/21) | 636 | 55 (25 - 79 | Prospective, non-randomized | > 18 years old, Thoracic X-ray or CT scan highly suspicious for lung cancer, PS ECOG 0 - 2absence of known or suspected pulmonary metastases | 267 | 51 | 14 | 304 |
| 5. Zhu, 2022 (20) | China | 916 (760/156) | 916 | 64 (19 - 87) | Prospective, non-randomized | > 18 years old clinical and radiology findings highly suspicious for lung cancer, surveillance and follow-up of patients after curative pulmonary cancer surgery | 724 | 19 | 66 | 107 |
| Author, Year | Image Bronchoscopy Technique | NBI System | Biopsy Instrument | No. Bronchoscopists | Lesion Characterization |
|---|---|---|---|---|---|
| 1. Vincent, 2007 (24) | Examined under WL and then with NBI mode | Flexible video bronchoscopy BF-Q180, Olympus EVIS EXERA II, CLV 180 light source, OEV-191H LCD monitor | Radial serrated-jaw forceps | 2 experienced bronchoscopists | Shibuya’s descriptors (dotted, tortuous and abrupt ending blood vessels). |
| 2. Bojan, 2009 (23) | Examined under WL and then with NBI mode | Flexible video bronchoscopy BF-1T180, Olympus EVIS LUCERA spectrum, Xenon light source CLV-260, SL19-inch LCD monitor OEV-191 | FB-22C- 1, oval biopsy forceps with spike | 2 experienced bronchoscopists | Shibuya’s descriptors (dotted, tortuous and abrupt ending blood vessels). |
| 3. Advani, 2018 (21) | Examined under WL and then with NBI mode | Not mentioned | Not mentioned | 2 bronchoscopists | Shibuya’s descriptors (dotted, tortuous and abrupt ending blood vessels). |
| 4. Zaric, 2009 (22) | Examined under WL and then with NBI mode | Flexible video bronchoscopy BF-1T280, Olympus EVIS LUCERA SPECTRUM, Xenon light source CLV-260SL, 19-inch LCD monitor OEV-191 | FB-22C-1, oval biopsy forceps with spike | Not mentioned | Shibuya’s descriptors (dotted, tortuous and abrupt ending blood vessels). |
| 5. Zhu, 2022 (20) | Examined under WL and then with NBI mode | Flexible video bronchoscopy BF-H290 NBI, Olympus EVIS LUCERA SPECTRUM, Xenon light source CV-260SL, 19-inch OEV-191LCD monitor | Not mentioned | 2 or 3 experienced bronchoscopists | Shibuya’s descriptors (dotted, tortuous, and abrupt ending blood vessels). |
4.3. Methodologic Heterogeneity
The quality of the studies is presented in Table 3. Most of the studies’ quality in this meta-analysis was good. All studies enrolled pro-diagnosed lung cancer subjects, who had previous clinical and radiological signs suggestive of lung cancer. Further, one study also presented abnormal sputum cytology results. Most of the sampling methods used were consecutive sampling. Additionally, Advani et al. allocated the subjects into two groups, NBI and WLB (21). Two studies aimed at assessing malignancy at the margins of airway tumors. Hence, they did not perform a tumor biopsy (22, 23). All studies reported the use of the NBI procedure as an index test without being combined with other image technologies. All biopsies conducted histopathological examinations as reference tests. Pathologists were blinded to bronchoscopic findings in all studies.
Abbreviations: L, low; H, high; U, unclear.
4.4. Diagnostic Accuracy of NBI Bronchoscopy
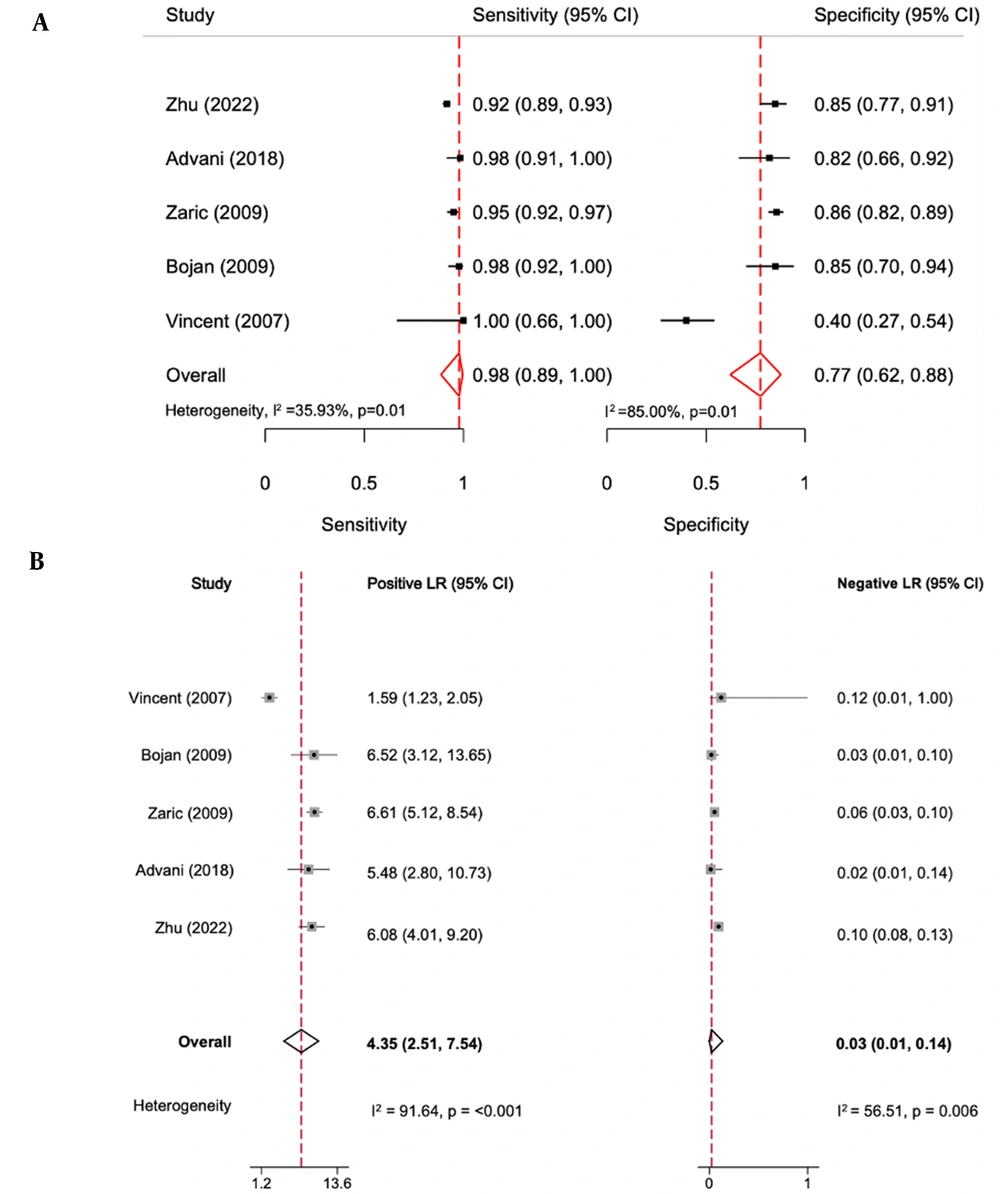
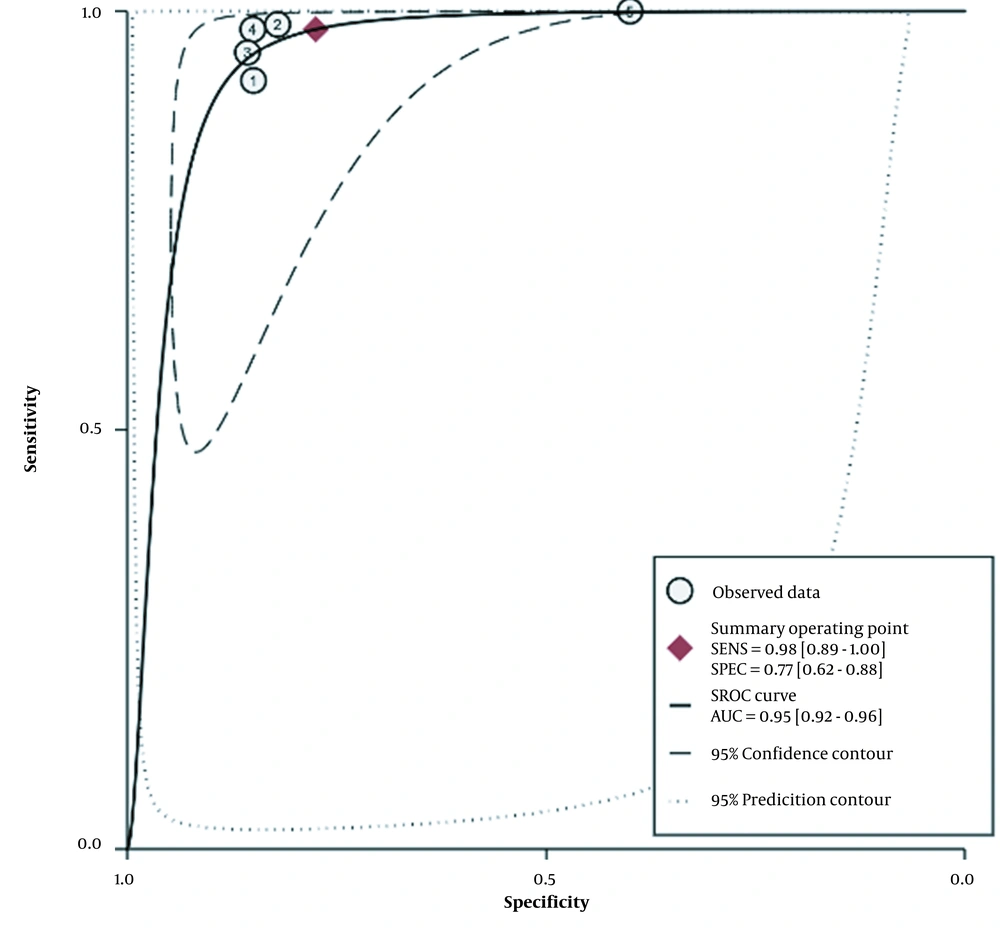
The diagnostic accuracy of NBI bronchoscopy is shown in Figure 2. The overall pooled sensitivity and specificity for NBI bronchoscopy for the early detection of airway cancer lesions were 98% (95% confidence interval [CI]: 89% - 100%) and 77% (95% CI: 62% - 88%), respectively. The heterogenicity I2 values for sensitivity and specificity were 35.93% and 85%, respectively, with a P-value of 0.001. The positive LR was 4.35 (95% CI: 2.51 - 7.54, P < 0.001), and the negative LR was 0.03 (95% CI: 0.01 - 0.14, P = 0.006). All the I2 values were ≥ 50%. The area under the receiver operating characteristic curve was 0.95 (95% CI: 0.92 - 0.96; Figure 3).
Forest plot of accuracy of narrow-band imaging (NBI) bronchoscopy for early detection of airway cancer lesions. (A) The sensitivity and specificity of NBI bronchoscopy for early detection of airway cancer lesions. (B) The positive likelihood ratio (LR +) and negative (LR -) NBI bronchoscopy for early detection of airway cancer lesions.
5. Discussion
In this meta-analysis, we found that the addition of NBI to standard bronchoscopy or WLB can improve performance test characteristics. Additionally, NBI bronchoscopy has a good diagnostic yield with high accuracy, high sensitivity, and sufficient specificity for detecting airway cancer lesions. Further, this good accuracy rate showed the great potential of NBI bronchoscopy imaging in determining both bronchial mucosa and submucosa pathological vascular patterns.
The NBI imaging system uses blue (415 nm) and green (540 nm) narrow light waves that produce high-resolution images of the vascular mucosa and submucosa. Additionally, the mechanism above can improve the contrast performance of an image (25). Some of the altered vascular pattern images that can be found in NBI examinations are increasing vessel growth and complex vascularization networks (12). The vascular pattern image is influenced by bronchial epithelium and vascular density abnormalities/differences. Previous studies have stated that this vascular pattern abnormality is associated with angiogenic squamous dysplasia (ASD) and lung cancer angiogenesis (11, 26).
All studies used Shibuya criteria to determine pathological morphological imaging on NBI bronchoscopy. However, several research groups have different criteria for describing abnormalities in the NBI video bronchoscopy. Vincent et al. determined abnormality in NBI bronchoscopy when a discordant appearance was found compared to WLI in terms of both increased capillary density and vascular pattern (24). Compared to Vincent et al., Herth et al. formulated the criteria for suspiciously malignant if there were at least 3 of the following abnormal vascular patterns, including dotted vessels, capillary loops, tortuous vessels, and abrupt ending vessels (27). Other studies represent almost the same terminology and criteria, consisting of dotted, tortuous, tortuous dilated, and spiral and screw vascular patterns (28). Moreover, previous studies tried to correlate the vascular pattern of the NBI video bronchoscopy imaging and its histology. The study reported about 27% to 37% of tortuous vasculature was associated with squamous cell carcinoma and high-grade dysplasia, as well as several other patterns of NBI vascular image pattern associated with high-grade lesions and preneoplastic airway lesions (29).
In this meta-analysis, the sensitivity was slightly higher and the specificity was lower (91% and 81%, respectively) than in the NBI bronchoscopy meta-analysis by Zhu et al. (30). However, the meta-analysis also included studies that performed autofluorescence technology (AFI) in addition to NBI bronchoscopy imaging (27, 31). Some studies have reported that the NBI procedure was comparable with AFI in the early diagnosis of lung cancer. However, the combination of NBI and AFI did not significantly improve the diagnostic value, which suggests that NBI and AFI are complementary tests (27, 31, 32). In addition, Chen et al. reported the sensitivity and specificity of AFI as 0.90 (95% CI: 0.84 - 0.93) and 0.56 (95% CI: 0.45 - 0.66), respectively. Our meta-analysis showed higher accuracy than Chen et al. (33).
The specificity of NBI in detecting airway malignancy as an NBI imaging classification criterion is related to Shibuya’s criteria. An analytical cross-sectional study reported that the vascular dotted pattern has a very high specificity for determining airway malignancies, including squamous and adenocarcinoma types. In contrast, compared to the dotted vascular pattern, the dilated tortuous vascular pattern has a higher sensitivity in detecting airway malignancy but has low specificity (34). Spiral and screw-type tumor vascular patterns are also more common in invasive airway tumors. Vascular tortuous vascularization patterns are more frequent in precancerous lesions of squamous dysplasia and ASD (12). Separate studies on the diagnostic accuracy of precancerous lesions of NBI bronchoscopy are still quite limited. Studies report that NBI sensitivity and specificity range from 65% to 74% and 59% to 67%, respectively (32).
5.1. Conclusions
Narrow-band imaging bronchoscopy can be used for detecting airway cancer lesions in suspected lung cancer patients. Although NBI image expertise is a subjective assessment, our study shows that NBI has good accuracy. Narrow-band imaging imaging will have a potential and significant role in future lung cancer, screening, diagnosis, and staging. More studies are necessary to confirm the diagnostic yield of NBI bronchoscopy, especially regarding the relationship between NBI vascular patterns and histopathology, as well as the accuracy of the detection of precancerous lesions.