1. Context
By 2021, it is estimated that prostate cancer is the most frequent malignancy in men. Also, it is the second leading cause of cancer deaths (1, 2). Prostate cancer occurs more commonly in aged men, and studies show that one-third of men in the third and fourth decades of life have histological evidence for prostate adenocarcinoma (3). Prostate tumors are associated with several factors, including age, heredity, diet, environment, and race. On the other hand, inflammation is an important factor in prostate disease and it may have an effective role in increasing the growth of tumor cells (4, 5).
Treatment for prostate cancer varies at various stages of the disease depending on the degree of the tumor and the estimated life expectancy. However, the ideal cure for this disease has not yet been found, and the effectiveness of treatments varies according to the progression of cancer (6-8). Common treatments for patients with prostate cancer are topical, radical prostatectomy, radiation therapy, hormone therapy, and the use of cryosurgery (6, 7). Other therapies such as angiogenesis, anti-angiogenesis, and immunotherapy are also considered today (8, 9).
Immune checkpoint inhibitors (ICIs), especially drugs that target PD-1 and PDL-1, improve treatment outcomes for advanced cancers, including non-small cell lung cancer (NSCLC), malignant melanoma (MM), urethral cancer, renal cell carcinoma (RCC), gastric cancer and cancer of head and neck. As a result, it has been considered by researchers as one of the most successful and effective treatments in reducing side effects, and according to the results of recent studies, it is better tolerated than chemotherapy (10, 11). ICIs are drugs that have been proposed in recent years as proposed treatments for prostate cancer (12-14). According to a 2021 review study by Venkatachalam et al., metastatic prostate cancer has been identified as a deadly disease with limited treatment options that immune suppression has dramatically changed the treatment outlook for a variety of cancers (15).
2. Objectives
In this systematic review and meta-analysis, we write an updated literature, summarizing available data and reporting practical suggestions about the efficacy and tolerability of ICIs in the treatment of prostate cancer, which may guide physicians in their current practice.
3. Data Sources
This study was registered with the code Prospero CRD42021252562 on the site www.crd.york.ac.uk on 26/05/2021.
3.1. Search Strategy
We conducted this systematic meta-analysis based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A systematic search of various databases including PubMed, Scopus, ClinicalTrial.gov, Web of Science, Cochrane Library, ScienceDirect, and Google Scholar was conducted from January 1, 1990, to January 1, 2022. We used MeSH terms to find exact keywords and ensure the completeness and accuracy of data retrieval. The following search terms used were prostate cancer, immunotherapy, ICIs, anti-programmed death-1 or anti-PD1, programmed death ligand-1, PD-L1 or anti-PD-L1, tolerability, and efficacy. With a search of the lists of references in articles included in this study, further relevant articles were obtained. Search data were included in EndNote X20 (Clarivate, London, UK).
4. Study Selection
The inclusion criteria of the study included: (1) prospective clinical trials, cross sectional, case-control, and case reports studies in patients with prostate cancer; (2) studies of the clinical efficacy and tolerability of ICIs treatments; (3) studies that measured overall survival (OS), objective response rate (ORR), or progression-free survival (PFS), and (4) English language studies. Exclusion criteria included (1) reviews, guidelines, letters, and news studies; (2) non-ICI treatment; (3) articles without available data or full text; and (4) duplicate publications or participants.
5. Data Extraction
Data extraction includes the author name, year of publication, study setting (hospitalized patients versus outpatients), study design, study methodology (the process of randomization, allocation, and blinding), study population, demographic characteristics of participants, details of intervention and comparator (dose, duration and route of administration), adverse events (AEs), and hazard ratios (HRs) with 95% CIs in OS and PFS and ORR (complete or partial response).
5.1. Risk for Bias and Quality of Evidence
The probable bias of publications was assessed with funnel plots and the Egger test. Two reviewers independently extracted key data from each eligible study. Any disagreement between two reviewers is resolved with a third author. The criteria provided by Cochrane are used to evaluate studies. Each study is accordingly classified into one of the groups’ exact or probably low risk of bias or exactly or probably the high risk of bias. The Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system is used to assess the quality of the expected outcomes.
5.2. Methodological Quality Assessment
Based on the Handbook of Cochrane for Systematic Reviews of Interventions (version 5.1.0), the methodological quality of included trials was assessed by 2 reviewers (H.S. and E.S.). Oppositions were resolved by a third reviewer (M.S.). Each trial was evaluated for 7 items: random sequence generation, concealed allocation, blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases; each item was rated as “high risk”, “low risk” or “unclear”.
5.3. Statistical Analysis
Stata version 17 (Stata Corporation, College Station, Texas US) and Review Manager Version 5.4 software were used to conduct statistical analyses. We used the HRs with corresponding 95% CI to assess the effect of ICIs on OS and PFS in patients with prostate cancer. We extracted the number of incidents and participants in each treatment arm to compute the pooled risk ratio (RR) for dichotomous outcomes such as adverse events (AEs). We used the random-effects model, using the restricted maximum likelihood estimator (RMLE) weighting method, which incorporates between-study variability into the calculations for pooling data. Heterogeneity was evaluated by Higgins I2 statistic. If the I2 statistic was 0% to 25%, suggesting very low heterogeneity, 25% to 50% suggesting low heterogeneity, 50% to 75% suggesting moderate heterogeneity, and higher than 75% suggesting high heterogeneity. Also, a chi-square test for heterogeneity was performed, and the P-value was presented. All comparisons were two-tailed, and 95% CI was described where applicable.
6. Results
6.1. Study Selection
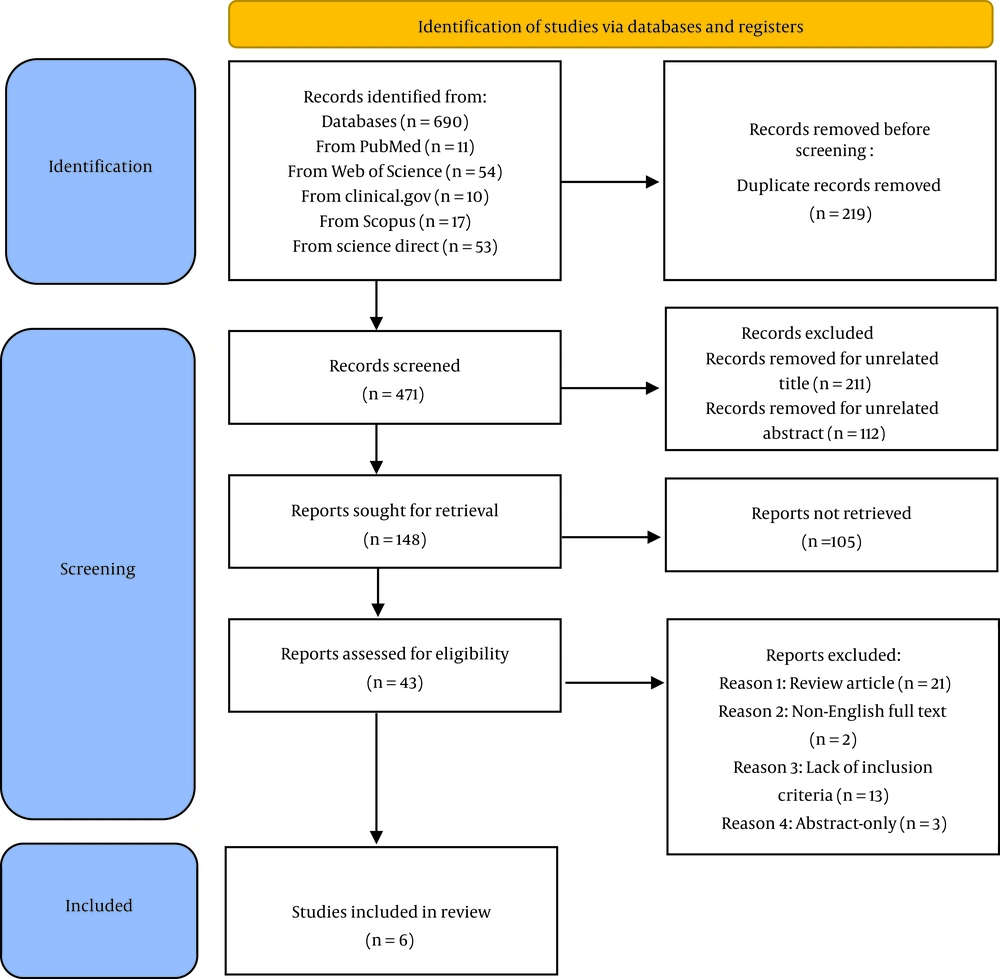
Initially, 690 records were identified through database searching. All studies were, then, imported into EndNote X9 software (Thomson Reuters) and 219 duplicates were removed. In addition, 323 studies were removed after the title and abstract screening process; thus, 43 were screened for eligibility more in detail. Finally, after a detailed title, abstract and full-text evaluation, 6 studies were included in this systematic review and meta-analysis. The flow diagram of the complete literature search and selection of studies is presented in Figure 1.
6.2. Study Characteristics
The characteristics of included studies were mentioned in Table 1. The ethnicity of the studied population varied as follows: United States (n = 5) and Canada (n = 1). All included studies were published between 2012 and 2021 in the English language. In general, 1 800 patients were examined with individual study sample sizes ranging from 23 to 799. The included studies were RCTs (n = 2) and non-randomized phase I clinical trials (n = 4). The included two RCTs involved 799 patients in the intervention and 602 patients in the control groups. They both evaluated radiotherapy combined with antieCTLA-4 antibodies (ipilimumab) versus radiotherapy with a placebo in patients with metastatic castration-resistant prostate cancer. A clinical trial in phase I, assessed BMS-936558, a fully human IgG4-blocking monoclonal antibody that acted for PD-1 in resistant prostate cancer patients. Another phase Ib trial assessed the effect of 10 mg/kg pembrolizumab every 2 weeks in patients with cytological or histologically recorded, advanced, or metastatic prostate adenocarcinoma locally.
| Study | Country | Study Design | Population | Sample Size | Intervention(s) | Control | Outcome, Measures | |
|---|---|---|---|---|---|---|---|---|
| Case | Control | |||||||
| Slovin et al. 2013 (16) | USA | Multicenter phase I/II study | Patients with metastatic castration-resistant prostate cancer | 34 | 16 | 10 mg/kg or at 3 or 10 mg/kg + radiotherapy | Ipilimumab every 3 weeks × 4 doses at 3, 5 | Adverse events, prostate-specific antigen (PSA) decline, and tumor response |
| Kwon et al. 2014 (17) | USA | Randomized clinical trial | Men with at least one bone metastasis from castration-resistant prostate cancer | 399 | 400 | Bone-directed radiotherapy followed by either Ipilimumab 10 mg/kg | Bone-directed radiotherapy followed by either placebo 10 mg/kg | Overall survival, progression-free survival, adverse events |
| Beer et al. 2017 (18) | USA | Randomized clinical trial | Asymptomatic or minimally symptomatic patients with chemotherapy-naive metastatic castration-resistant prostate cancer without visceral metastases | 400 | 202 | Ipilimumab 10 mg/kg every 3 weeks for up to four doses | Placebo 10 mg/kg every 3 weeks for up to four doses | Overall survival, progression-free survival, adverse events |
| Topalian et al. 2012 (19) | USA | Phase I clinical trial | Patients with advanced melanoma, non–small-cell lung cancer, castration-resistant prostate cancer, or renal-cell or colorectal cancer | 296 | - | BMS-936558, a fully human igg4-blocking monoclonal antibody directed against PD-1, | - | Response rates, adverse events |
| Hansen et al. 2018 (20) | Canada. | Phase Ib trial | Histologically or cytologically documented, locally advanced or metastatic prostate adenocarcinoma that was incurable and for which standard therapy was ineffective or not considered appropriate; at least one measurable lesion at baseline | 23 | - | Pembrolizumab 10 mg/kg every 2 weeks until disease, Progression or intolerable toxicity for up to 24 months | - | Response rates, adverse events |
| Shenderov et al. 2021 (21) | USA | A phase‐2 nonrandomized clinical trial | Histologically confirmed, progressive, with detectable AR‐V7 transcripts using the Johns Hopkins CTC‐based clinical‐grade AR‐V7 assay | 15 | 15 | Nivolumab (3 mg/kg) plus Ipilimumab (1 mg/kg), | Ipilimumab plus Nivolumab and Enzalutamide | Prostate‐specific antigen (PSA) response rate time‐to‐PSA‐progression‐free survival (PSA‐PFS), time-to-clinical/ radiographic‐PFS, objective response rate (ORR), PFS lasting greater than 24 weeks, and overall survival (OS). |
Key Characteristics of Included Studies
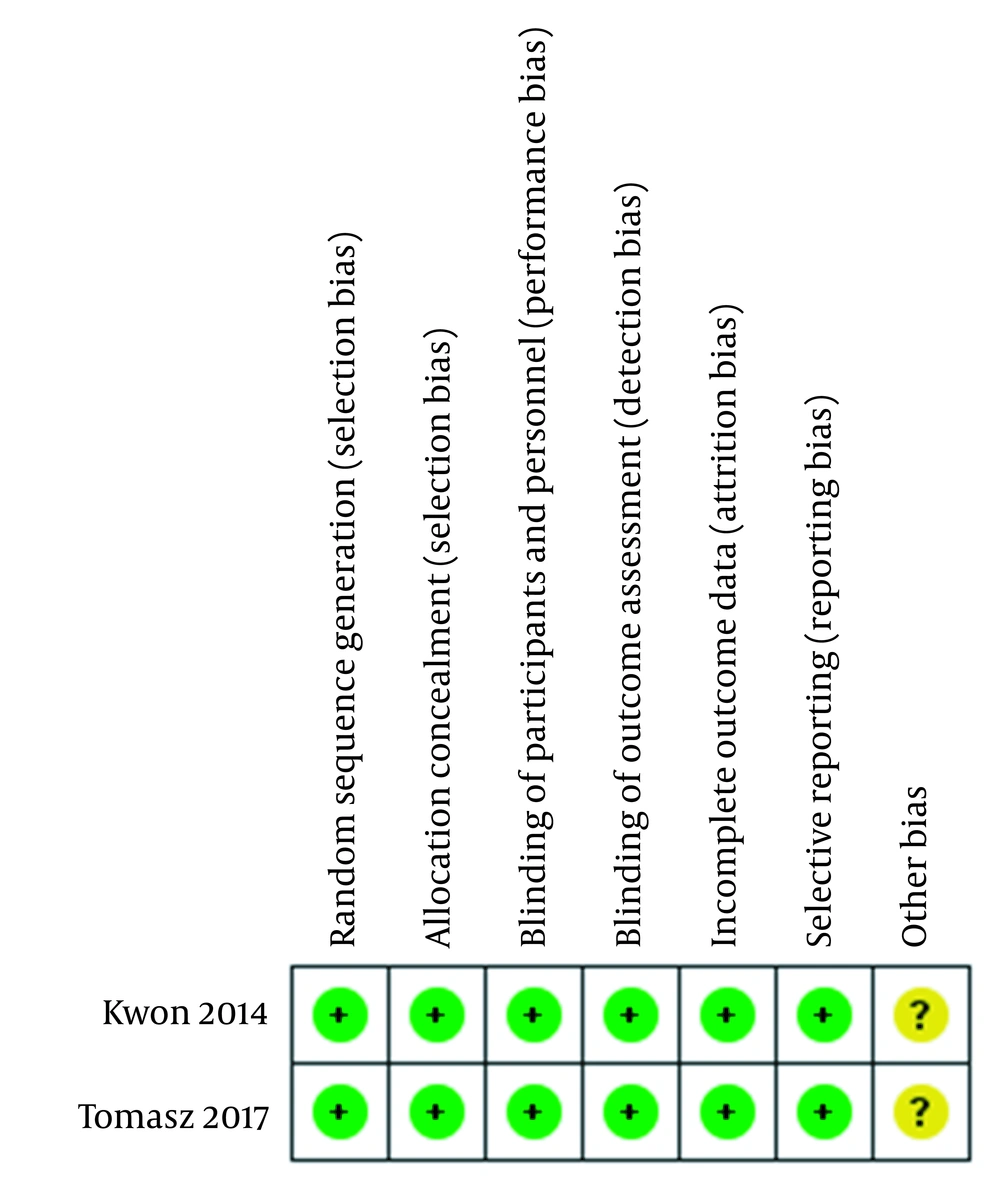
In a multicenter phase I/II study, 33 metastatic castration-resistant prostate cancer patients received ipilimumab 4 doses at 3, 5, or 10 mg/kg every 3 weeks accompanied by radiotherapy. Also in another phase‐2 nonrandomized clinical trial, nivolumab plus ipilimumab was compared with ipilimumab plus nivolumab and enzalutamide in patients with histologically confirmed progressive prostate cancer. The risk of bias summary demonstrated that the methodological quality of these trials was completely desirable (Figure 2).
All participants in the two included trials were randomly allocated to groups, using an adequate allocation procedure. The allocation concealment was at low risk in the two trials. The trials used subject masking, and the blinding of participants and personnel was at low risk for performance and detection bias. In addition, the trials provided an intention-to-treat (ITT) analysis. So, they were at low risk for attrition bias. Reporting bias for all trials was low risk. Last but not least, the trials were judged to be unclear for other biases.
6.3. Overall Survival
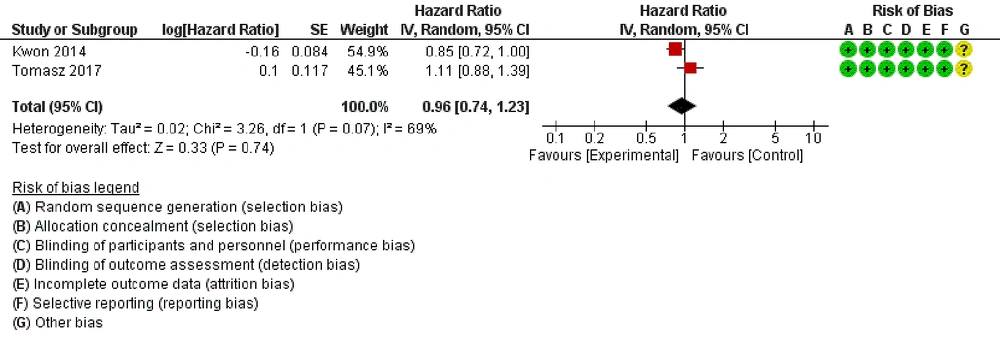
Overall survival was reported in 2 trials. The results of this meta-analysis showed no significant difference in the risk of death for comparing ICI + radiotherapy with placebo+ radiotherapy (HR: 0.96, 95% CI: 0.74, 1.23, P = 0.74; Figure 3). High heterogeneity was detected between trials (I2 = 69%, P = 0.07).
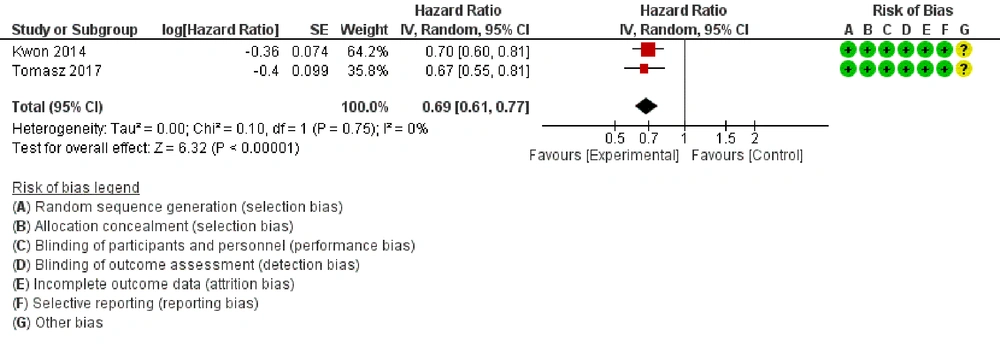
6.4. Progression-free Survival
Pooling results from two RCTs, that compared the effect of ICI versus placebo, suggested that the risk of death was less in the joint ICI + radiotherapy group compared with the group that received just radiotherapy (HR: 0.69, 95% CI: 0.61, 0.77, P < 0.001; Figure 4). There was no heterogeneity between trials (I2 = 0%, P = 0.75).
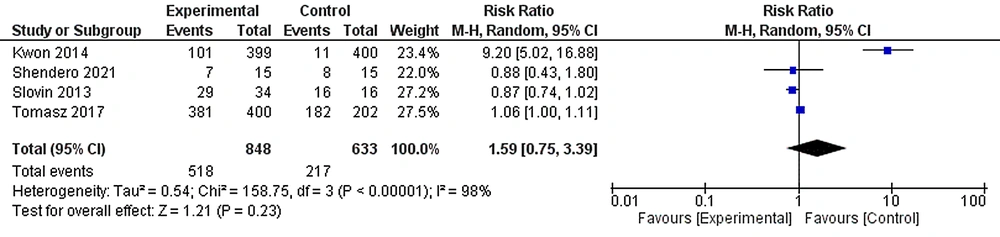
6.5. Tolerability
We extracted some AEs of any grade in experimental and control groups in all studies. AEs data were get from 4 included studies with 1481 patients (848 cases and 633 controls). The most common AEs occurred in 518 (61.08%) patients in the experimental group and 217 (34.28%) patients in the control group. The random effects forest plot for all included studies showed a non-significant difference between groups considering the overall prevalence of treatment-associated AEs (RR: 1.59, 95% CI: 0.75, 3.39, I2 = 98%; Figure 5).
7. Discussion
Immune checkpoints such as PD1 and CTLA-4, are noticed as goal antigens for undertaking immunotherapy own to the immune-inhibitory functions and their high expression in tumor microenvironment immune cells (22, 23). In recent years, immunotherapy has drawn many considerations in patients suffering from PCa. Although new treatments such as hormone therapy and radiotherapy are effective for localized cancer, they are not enough for advanced prostate cancer. One of the most important arms of immunotherapy in the treatment of cancer is ICIs. ICIs are an effective treatment for several types of cancer including prostate cancer (24-26). In the therapeutic process, ICIs as specific antibodies bind to inhibitory receptors and block them to protect the activation of T cells against cancer cells (27, 28). Due to the growing importance of these ICIs in prolonging the survival of cancer patients along with conventional therapies, in the current study, a meta-analysis was performed and the association of ICIs plus conventional therapies with overall survival, PFS, and tolerability in patients with prostate cancer was determined. Our study is the initial meta-analysis study to collate the survival, efficacy, and safety of ICIs in prostate cancer patients with standard radiotherapy to date.
Most clinical trials performed are combined therapies, such as ICIs + radiotherapy in advanced prostate cancer (16). However, these therapies in the treatment of advanced prostate cancer demonstrated modest success (17, 18). Some issues might be predicted, such as selecting the right combination of the drug with conventional therapies as well as optimizing the drug dose, and ameliorating adverse effects and toxicities (17-21).
There was no significant difference between the two studied RCTs in the overall survival of patients with prostate cancer treated ICI + radiotherapy when compared with placebo+ radiotherapy. However, the PFS from two RCTs was longer in patients treated with ICI + radiotherapy compared with the radiotherapy alone group, indicating the possible efficacy of ICIs associated with radiotherapy as a combination therapy for tumor good prognosis and the importance of ICIs in reducing disease progression. A different expression level of the immune checkpoints such as B7-H3, PD-L1, PD-L2, and CTLA-4 was found in circulating tumor cells (CTCs) in metastatic prostate cancer patients, suggesting that ICIs may have the benefit in some cases with metastatic prostate cancer but not all (29).
Combination of immunotherapy especially ICIs with chemo-radiotherapy can be a considerable option to make more studies in the future on many solid tumors because now only a subset of cancer patients benefits from ICIs. Although synergistic effects of ICIs with radiotherapy, chemotherapy, and tumor-specific antigen-targeted therapy were validated in different kinds of cancer (16, 21), some questions remain unanswered, and future tails should be conducted to the best strategy in terms of duration, dose, timing and series of injections.
The most important pathological parameter in the diagnosis of patients with prostate cancer is the Gleason score. Patients with advanced prostate cancer usually have high Gleason scores (7 (4 + 3), 8, 9, and 10) and there is a strong and direct relationship between increased Gleason scores and poor prognosis (30). However, this study failed to perform the Gleason degree with the survival of patients treated with ICIs. Another limitation was the lack of meta-analysis capability of biochemical recurrence (BCR) after treatment with ICIs. After early treatments for patients with localized PCa, enhanced levels of PSA (prostate-specific antigen) in the blood are evaluated as BCR. Intermittent measurement of the PSA helps physicians diagnose relapse after initial treatment and increased PSA serum level is the most factor for disease recurrence after surgery.
This meta-analysis had some limitations. All of the included studies were performed in the same location and other countries did not have published results in this field. Besides, some studies did not report AE, PSA response, and tumor response. Furthermore, only a few articles were eligible for the criteria of selection.
7.1. Conclusions
According to the results of our study, the use of safety checkpoint inhibitors has been associated with improved survival without progression, but their effect on increasing overall survival has not been confirmed. Also, the side effects indicate that the incidence of toxic and fatal side effects was not higher in patients receiving this medication regimen. Reported side effects can also be relieved with routine treatments or discontinuation of treatment with this class of drugs. However, due to the low number of articles included in the present meta-analysis, it is not possible to comment with certainty, and more extensive studies are needed in the coming years.