1. Background
Toxoplasma gondii is an obligatory intracellular pathogen that infects approximately one-third of the world’s population and in most people mainly without complication and clinical symptoms (1). Domestic cats are the definitive host, and all warm-blooded animals and humans are intermediate hosts of the parasite. The infection can happen by ingesting cysts from raw or uncooked meat or ingesting oocysts from water or foods contaminated with cat feces (2-4). The parasite has 3 infective forms, including tachyzoite, bradyzoite in tissue cysts, and sporozoite in sporulated oocysts (5).
According to the World Health Organization (WHO) report, breast cancer is the most common cancer and the main cause of death by cancer among women all over the world (6). Despite advances in cancer treatment including surgery, chemotherapy, and radiotherapy, the survival rate for many cancers is very low. To improve cancer therapy, stimulating effective immune responses against cancer has been established in the last decades (7). Microorganisms such as Toxoplasma gondii may be good candidates to stimulate the immune system against cancer growth (8). Anti-cancer activities of this parasite have been investigated in previous studies (9-12). Toxoplasma gondii induces strong polarization of Th1 responses in its host, with an augment in IFN-γ and IL-12 production (13, 14). Baird et al. showed that T. gondii can produce anti-tumor immunity against ovarian cancer (15). Also, intertumoral administration of a weakened species of T. gondii in a melanoma model cancer stimulated the anti-tumor immune response, mediated by CD8+ T cells and NK cells (16). Treatment of mice with Toxoplasma lysate antigen in the murine sarcoma-180 tumor model has been considered and results in a decrease of both tumor size and CD31 expression (11). Kim et al. observed that infection with T. gondii leads to the creation of a strong cell-mediated immune response in the laboratory mouse host (10). In addition, mice immunization with dendritic cells matured in the presence of T. gondii-derived profilin-like protein augmented the activity of cytotoxic T cells and so reduced the melanoma and fibrosarcoma tumor growth (17). Mohamadi et al. indicated that rabbit anti-T. gondii antiserum selectively reacts with the surface of murine melanoma and breast cancer cells (4T1) and not with normal mouse spleen lymphocytes (12).
2. Objectives
To find out whether other breast cancer cell lines react with human anti-Toxoplasma antibodies or not, in the present work, the reaction between Toxoplasma-positive and negative human sera with two breast cancer cell lines 4T1 (mouse origin) and MCF7 (human origin) has been investigated.
3. Methods
In this case-control work, the study population consisted of two breast cancer cell lines (4T1 and MCF7) and sera of patients referred to clinical labs for Toxoplasma infection examination. For this purpose, murine breast cancer cell line 4T1 and human breast cancer cell line MCF7 were purchased from Pasture Institute, Cell Bank of Iran (Tehran, Iran).
Spare serum samples of patients referred to different clinical labs for examination of Toxoplasmosis infection were collected. The serums were, then, transferred to the Department of Parasitology Lab at Isfahan University of Medical Sciences and kept at -20°C until needed. All collected sera were, then, tested, using ELISA Kit for the detection of anti-Toxoplasma IgG antibodies. According to the results of ELISA test including the OD of samples and the kit cut-off, the sera were classified as Toxoplasma-positive and Toxoplasma-negative samples.
3.1. Cell Culture
4T1 and MCF7 cell lines were cultured in RPMI-1640 medium (Roswell Park Memorial Institute Medium 1640) supplement with 10% fetal bovine serum (FBS) with 1% antibiotics mixture containing 100 IU/mL penicillin and 100 IU/mL streptomycin and incubated in a humidified atmosphere with 5% CO2 in a 37°C incubator.
3.2. ELISA
Collected serum samples were examined for the presence of anti-toxoplasma IgG, using the ELISA method. For this purpose, Toxoplasma IgG ELISA kit (Pishtazteb) was purchased and the procedure was performed according to the kit instructions.
3.3. Reaction of Serum with the Cancer Cell Lines Using Flow Cytometer Equipment
Cancer cells 4T1 and MCF7 were harvested from the culture medium by trypsin and washed 3 times with PBS. The cells were, then, counted and for each cell line, 16 tubes containing 105 cells were set up. Tubes 1 to 8 were treated with Toxoplasma-positive sera as the case group. Tubes 9 to 16 were treated with Toxoplasma-negative sera as the control group. After 1 hour of incubation, the tubes received 3 washes and, then, secondary antibodies (Goat Anti Human IgG-FITC from Bio Legend company) conjugated with fluorescein isothiocyanate were added. The tubes were washed again 3 times. The reaction of antibodies with the surface of cells was analyzed, using a flow cytometer equipment (BD Cell Quest Pro software version 6). The experiment was, then, repeated 3 times with appropriate positive and negative controls.
3.4. Statistical Analysis
The t-test was used for statistical analysis. In the comparison of means, P < 0.05 was considered meaningful.
4. Results
4.1. Results of ELISA Test
Sera collected from clinical labs were subjected to ELISA test; 30 sera with OD more than 1 were considered as Toxoplasma-positive and 30 sera with OD less than 0.2 were considered as Toxoplasma-negative.
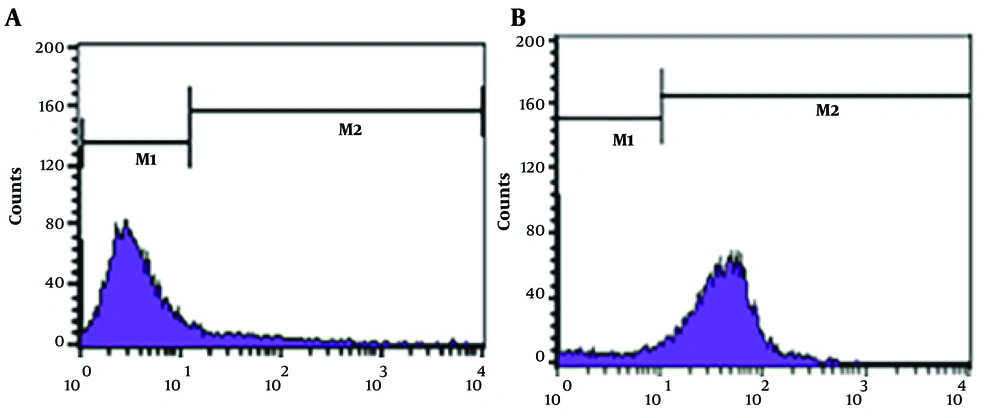
4.2. The Reaction of Toxoplasma-Positive Sera with the Surface of 4T1 Breast Cancer Cells
The reaction between Toxoplasma-positive sera and Toxoplasma-negative sera with 4T1 cell surface was investigated, using the flow cytometry method. In this experiment, reacted cells with Toxoplasma-positive sera stained with fluorescein isothiocyanate and shifted into the M2 area in the flow cytometry plot while untreated cells remained in the M2 area (Figure 1). So, Toxoplasma-positive sera exhibited a marked reaction with 4T1 cells, while the reaction with Toxoplasma-negative sera was weak. In this experiment, cells without serum treatment and treated cells without secondary antibodies were used as negative controls. This experiment was repeated 3 times. Analysis of experimental repeats indicated that the difference between M2 cells (reactive) in Toxoplasma-positive and Toxoplasma-negative treated groups was statistically significant (P < 0.05). Details of these experiments have been presented in Table 1.
| 4T1 | Toxoplasma Positive Sera | Toxoplasma Negative Sera |
|---|---|---|
| M1 | 29.54 ± 3.36 | 84.98 ± 2.32 |
| M2 | 70.28 ± 3.42 | 11.55 ± 3.04 |
Reaction of Toxoplasma positive and negative sera with the surface of MCF7 breast cancer cells
The reaction of Toxoplasma positive and negative human sera with the surface of MCF7 cells was investigated. In this experiment, MCF7-reacted cells with Toxoplasma-positive sera stained with fluorescein isothiocyanate and shifted into the M2 area in the flow cytometry plot while untreated cells remained in the M2 area (Figure 2). So, Toxoplasma-positive sera exhibited a marked reaction with MCF7 cells, while the reaction with Toxoplasma-negative sera was weak. The experiment was repeated 3 times. In these experiments, cells without serum treatment or secondary antibody treatment were used as negative controls. Analysis of experimental repeats indicated that the difference in M2 (reactive) cells between Toxoplasma positive and negative sera was statistically significant (P < 0.05). Detailed results are shown in Table 2.
| MCF7 | Toxoplasma Positive Sera | Toxoplasma Negative Sera |
|---|---|---|
| M1 | 17.53 ± 2.245 | 71.58 ± 3.42 |
| M2 | 82.66 ± 2.207 | 25.67 ± 3.45 |
5. Discussion
The results of the present work indicated that Toxoplasma-positive human sera reacted with the surface of murine breast cancer cells (4T1) and human breast cancer cells (MCF7), while the reaction of these cells with Toxoplasma-negative sera was weak. These results indicate that anti-Toxoplasma antibody reacts with the surface of both 4T1 and MCF7 breast cancer cells. In agreement with these results, we have already shown that rabbit anti-Toxoplasma serum reacted selectively with the surface of 4T1 cells but not with the surface of mouse spleen cells (12).
The reaction of anti-Toxoplasma antibodies with the surface of breast cancer cells may be due to the existence of shared antigens between this parasite and the cancer cells. In line with this hypothesis, in previous studies, the existence of common antigens between certain parasites and cancers has been shown (18, 19). In this regard, Salanti et al. displayed that a glycosaminoglycan protein of Plasmodium falciparum binds selectively to cancer cells (20). Also, attenuated T. gondii Me49 induced a strong cell-mediated antitumor immune response causing immune protection against primary cancer development (21).
Cancer cells' unusual mucines, such as the Tn, sialyl-Tn, or TF antigens, have important roles in cell adhesion, metastasis, and invasion of malignant cells (22). Also, Parasite glycans have important functions in the interaction of parasites and their hosts (23). It has been shown that glycan structures present in cancers were expressed by helminths, indicating the presence of shared antigens between parasites and cancers. As an example, both Schistosoma mansoni adult worms and schistosomula produce Tn antigen, which is expressed in cancer cells (24). Expression of the Tn antigen in adult and larval stages of Echinococcus granulosus has been shown in another work (25). Tk antigen, which is expressed at the surface of human colorectal carcinomas, has also been found in Taenia crassiceps, Mesocestoides vogae, and Taenia hydatigena (26). Other molecules such as sialyl Tn antigens and TF antigens have also been recognized as common antigens between cancers and parasites (25, 27-30). In another work, it has been shown that mucin-like O-glycans antigen may induce an anti-cancer effect through activation of Th-1-polarized immune response (31).
In support of the presence of shared antigens between cancer cells and parasites, in another work, Shakibapour et al. using mass spectrometry analysis showed that hydatid cyst wall 27/28 kDa band has numbers of antigens with a high degree of homology with cancer cells (32). Daneshpour et al. also showed that antisera raised against hydatid cysts cross-reacted with excretory-secretory products of cancer cells. They also reported a cross-reaction between sera of breast cancer patients and antigens of hydatid cysts (33). Finally, Sharafi et al. showed that sera of patients with breast cancer reacted with a No glycosylated 27 KDa band antigen of hydatid cyst (34).
The effect of the interaction of Toxoplasma-positive sera with epitopes on the surface of cancer cells in the present study remains unclear. However, the attachment of the antibody to the cancer cells' surface may have some mortal effects on the cancer cells. Traditionally in cancer chemotherapy, cytotoxic agents are used to kill cancer cells. However, these agents have very little or no specificity to cancer cells. The non-specific action of the cancer drugs usually results in severe undesirable side effects (34). Therefore, finding a drug delivery protocol that can selectively impair cancer cells with no damage to normal cells would be very important and useful. In this regard, MA immunoconjugates were used to deliver drugs to tumors selectively (35). In this context, in our work, we showed that anti- anti-toxoplasma antibodies react with the surface of cancer cells. So, anti-Toxoplasma antibodies may be used in the future for selective cancer immunotherapy.
5.1. Conclusions
Human anti-toxoplasma positive but not negative ones reacted strongly with the surface of breast cancer cell lines. These reactive antibodies may be used for selective cancer immunotherapy in the future.