1. Background
Despite the development of various early diagnostic methods along with effective treatment approaches such as surgery and chemotherapy in the treatment of colorectal cancer (CRC), patients are facing clinical morbidity and mortality in the world to the extent that this cancer is known as the third most deadly cancer in the world (1). The mortality rate of CRCs is projected to increase due to the extraordinary global trend in the aging of the population and the reflective adverse effects of many lifestyle-related factors (2). The 5-year overall survival for cancer confined to the colon for stage I and stage II is 95% and 82%, respectively. Due to the lack of early diagnosis of this disease, especially in the early stages due to the lack of specific manifestations, the losses caused by this disease sometimes go beyond this. Hence, early recognition of CRC, before the stages of promotion/progression, is a main challenge for achieving an effective long-term consequence and a major problem in the clinical approach (3). Nowadays, different advanced modalities are employed to diagnose disease stage and its progression including colonoscopy, flexible sigmoidoscopy, and histological assessment; however, the invasive nature, pre-procedure preparation, and contraindications of these procedures have led to a decrease in the willingness of patients and even doctors to use these methods (4). In this regard, the current studies are focusing on non-invasive specific biomarkers that can detect CRC before reaching advanced stages.
Angiogenesis is a fundamental process necessary for tumor progression (5). In other words, the detection of new angiogenesis due to tumor tissue hypoxia can effectively predict tumor growth rate. Therefore, tracking the occurrence of angiogenesis in tumoral tissue, especially through increasing the expression of specific markers related to angiogenesis, can be an accurate and useful marker in predicting tumor growth. One of these specific biomarkers reflecting tumor angiogenesis is platelet-endothelial cell adhesion molecule-1 (PECAM-1) or CD31 belonging to the immunoglobulin superfamily (6). This marker is a cell membrane glycoprotein, whose high expression is in line with increasing tumor microvessel density and, as a result, the expression of tumor angiogenesis (7). CD31 is now suggested as a blood vessel invasion marker and, thus, can be applied for tracking tumor progression and even metastasis (8).
2. Objectives
We aimed at evaluating the association of CD31 expression with the likelihood of microvascular invasion and other abnormal histopathological findings with CRC in our society to predict aggressive behaviors.
3. Methods
In our cross-sectional study, the pathology numbers of 50 samples that were diagnosed with colorectal cancer in their pathology report during 2021 and 2022 were identified by searching the information system of Sina Hospital in Tehran. The inclusion criteria were any established CRC (obstructive or perforated) from total or partial colectomy or proctectomy. The excluding criteria were patients, who did not have appropriate paraffin blocks for IHC staining or received neoadjuvant therapy.
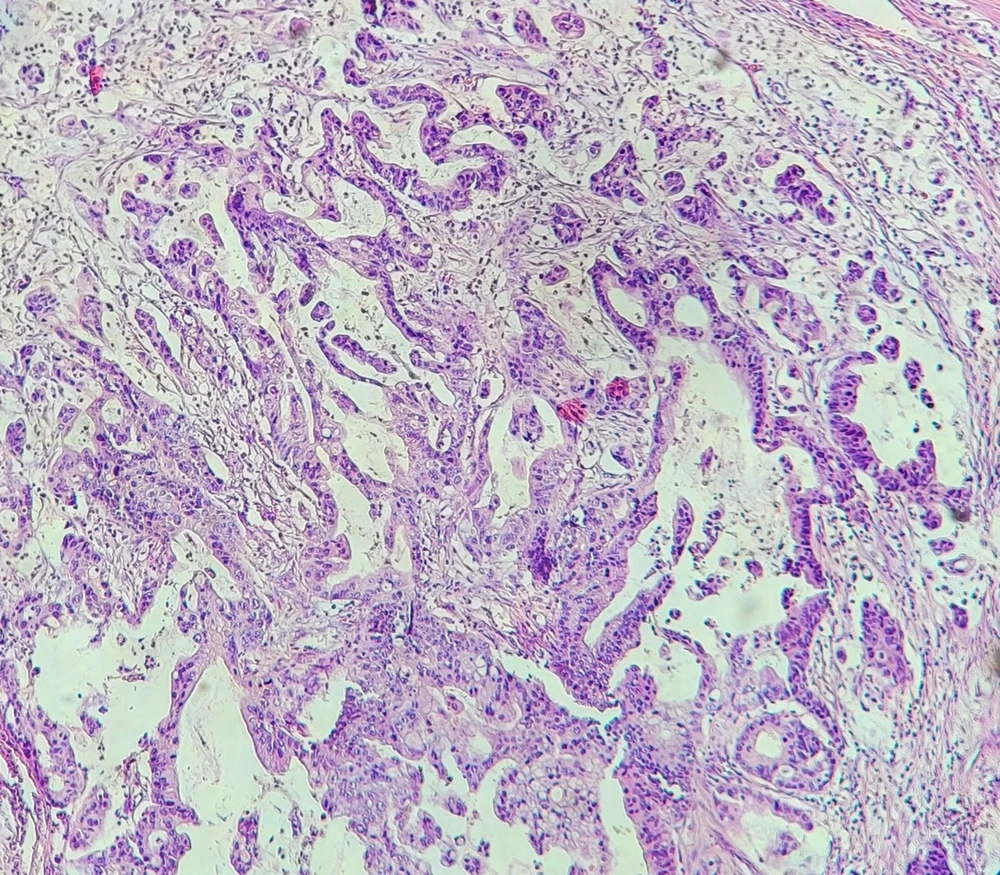
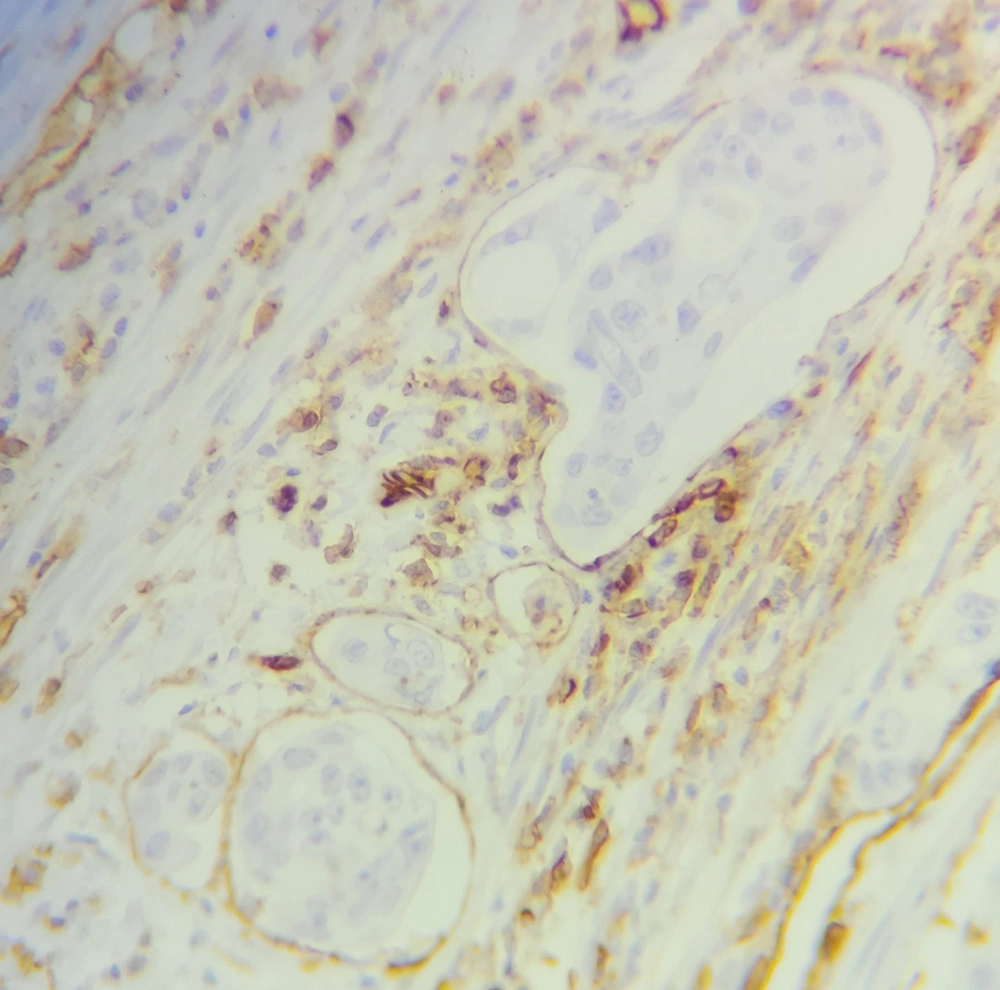
The baseline information required to conduct the study including the demographic and clinical information of the patients was extracted from their hospital records. Then, the paraffin blocks and hematoxylin and eosin (H&E) slides of these samples were extracted from the archives of the pathology department of the hospital, and after reviewing the H&E slides, in addition to confirming the diagnosis of colorectal adenocarcinoma, the presence of microvascular invasion was also examined by a pathologist (Figure 1). Also, a suitable paraffin block is selected for immunohistochemical staining (IHC). IHC using CD31 monoclonal antibody was performed for assessing CD31 positivity (Figure 2). To perform IHC, tissue sections with a thickness of 4 μm were prepared from paraffin blocks on a glass slide and, then, placed in an autoclave at 60°C for one night. Then, in the step of deparaffinization and hydration, the samples were placed 3 times in xylenol for 15 minutes each time, twice in 100% alcohol for 10 minutes each time, and finally twice in 96% alcohol for 10 minutes each time. Next, the samples were washed with distilled water and placed in an autoclave at 2 bar pressure and 95 degrees Celsius for 20 minutes with 1.5 cc of EDTA buffer with pH = 8 for antigen retrieval. Again, the slices were washed 2 to 3 times with distilled water and kept at room temperature for 20 minutes. Then, using a peroxidase solution, internal peroxidases were blocked for 10 minutes. In the next step, samples were placed in contact with CD31 monoclonal antibody for 10 minutes and, then, washed with TBS buffer and a secondary antibody was added. After 30 minutes, it was washed again with TBS buffer, and DAB chromogen was exposed to the samples for 10 minutes. In the end, the samples were washed in running water and stained with hematoxylin, and placed in bicarbonate for 30 seconds. After dehydrating, the samples were ready for mounting and microscopic examination. Finally, the stained slides were observed by a pathologist, and the presence of tumor cells in the spaces covered by CD31-positive cells was considered a microvascular invasion.
SPSS software version 21 was used for the statistical analysis of data. The results for quantitative variables were expressed as mean and standard deviation (mean ± SD) and for qualitative variables as percentage and frequency. Comparison between quantitative variables between two groups was done by t-test or Mann-Whitney test. Comparison between qualitative variables was also done, using the chi-square test or Fisher's exact test. The multivariable logistic regression model was used to test the association of CD31 positivity with tumor characteristics with the presence of baseline parameters such as sex, age, and tumor location. Differences were considered statistically significant when P < 0.05.
4. Results
In total, 50 patients suffering from colorectal cancers (31 located in the colon and 19 located in the rectum) were included in the study. Baseline characteristics are shown in Table 1. The median age of evaluated patients was 59.58 ± 13.56 years ranged 25 to 91 years and 54.0% were male. Regarding tumor grade, 66%, 28.0%, and 6.0% were graded as I, II, and III, respectively. The mean size of tumor mass was 4.65 ± 2.11 cm. Regarding tumor invasion status, neural invasion was reported in 10.0% and vascular invasion in 24.0%. Also, lymph node involvement was revealed in 36.0%.
| Variables | Values |
|---|---|
| Male gender | 27 (54.0) |
| Age (y) | 59.58 ± 13.56 |
| Tumor grade | |
| I | 33 (66.0) |
| II | 14 (28.0) |
| III | 3 (6.0) |
| Tumor stage | |
| I | 4 (8.0) |
| II | 11 (22.0) |
| III | 34 (68.0) |
| IV | 1 (2.0) |
| The mean size of the tumor (cm) | 4.65 ± 2.11 |
| Lymph node involvement | 18 (36.0) |
| Neural invasion | 5 (10.0) |
| Vascular invasion | 12 (24.0) |
| CD31 positivity | 17 (34.0) |
aValues are expressed as Mean ± SD or No. (%).
Overall, CD31 positivity was found in 34.0% of cases 35.5% in colon masses, and 31.6% in rectal masses. As shown in Table 2, those patients with positive CD31 had significantly higher tumor grades (P < 0.045). The prevalence rate of vascular invasion was significantly higher in patients with positive CD31 compared to those with negative CD31 states (47.1% versus 12.1%, P = 0.006). Also, the rate of lymph node involvement in the groups with positive and negative CD31 was 58.8% and 24.2%, respectively, indicating a significant difference (P = 0.016). Though, CD31 positivity was not related to tumor location, tumor stage, or size. Considering two subgroups of patients with colon and rectal masses, the association of vascular invasion and CD31 positivity was limited to the masses located in the colon, not in the rectum. In this regard, in the subgroup of patients with colon mass, the rate of vascular invasion in positive and negative CD31 status was 63.6% and 15.0%, respectively, indicating a significant difference (P = 0.006); however, there was no difference in vascular invasion rate in positive and negative CD31 condition in the subgroup with rectal mass (16.7% versus 7.7%, P = 0.554).
| Characteristics | Positive CD31 | Negative CD31 | P Value |
|---|---|---|---|
| Male gender | 10 (58.8) | 17 (51.5) | 0.623 |
| Age (y) | 63.47 ± 13.99 | 57.58 ± 13.09 | 0.147 |
| Tumor grade | 0.045 | ||
| I | 10 (58.8) | 23 (69.7) | |
| II | 4 (23.5) | 10 (30.3) | |
| III | 3 (17.6) | 0 (0.0) | |
| Tumor stage | 0.256 | ||
| I | 0 (0.0) | 4 (12.1) | |
| II | 4 (23.5) | 7 (21.2) | |
| III | 12 (70.6) | 22 (66.7) | |
| IV | 1 (5.9) | 0 (0.0) | |
| The size of the tumor (cm) | 4.98 ± 1.83 | 4.48 ± 2.25 | 0.431 |
| Lymph node involvement | 10 (58.8) | 8 (24.2) | 0.016 |
| Neural invasion | 2 (11.8) | 3 (9.1) | 0.765 |
| Vascular invasion | 8 (47.1) | 4 (12.1) | 0.006 |
aValues are expressed as Mean ± SD or No. (%).
In a multivariable logistic regression model with the presence of baseline parameters (Table 3), CD31 positivity was shown to be associated with the risk for vascular invasion, and the presence of positive CD31 was associated with a 6-fold increase in the risk of vascular invasion in colon cancers. Also, in a similar model, CD31 positivity could predict an increased likelihood of lymph node involvement by 6.5 times.
| Variables | Beta | P Value | OR | 95.0%CI for OR | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| CD31 positivity | 1.826 | 0.020 | 6.211 | 1.336 | 28.571 |
| Gender | -0.634 | 0.438 | 0.530 | 0.107 | 2.632 |
| Age | 0.025 | 0.373 | 1.026 | 0.970 | 1.084 |
| Location | -1.782 | 0.070 | 0.168 | 0.024 | 1.159 |
a Hosmer-Lemeshow goodness of fit: Chi-square = 6.135, P = 0.632
5. Discussion
Tumor angiogenesis is a necessary and marked step for cancer progression. Angiogenesis due to a hypoxic state of tumor cells provides the possibility of tumor cells’ vascular invasion and, thus, the promotion of metastasis (9). Biomarkers expression and histopathological findings are used to predict vascular invasions by tumor cells, metastasis, and response to target therapy (10). Some specific endothelial and vascular markers have been recently identified to be linked to tumor angiogenesis such as CD31, CD34, and CD105 (11). In other words, such biomarkers can effectively predict the vascular status of malignant tumors. As indicated in the current study, the presence of CD31 assessed by IHC was closely associated with increasing microvascular invasion in colon cancer. Because radiation can be a trigger for angiogenesis and patients who received neoadjuvant therapy were excluded from the study and included only low-stage rectum cancer (12), we detected only a correlation between CD31 positivity and vascular invasion in colon cancer. In other words, by tracking CD31 expression in confirmed cases of this cancer, it is possible to predict more invasive and, thus, metastatic patterns of cancer. It is obvious by detecting over-expression of CD31 in colon tumor cells, predicting the overall survival of the patients can be also possible. It has been previously demonstrated that increased microvascular density can be a powerful marker for advanced tumor stages and shorter overall survival of affected patients (9, 11). CD31 expression was significantly correlated with most vascular endothelial cell markers. Tumors with high CD31 expression are associated with increased vascular stability and immune response pathways, and these tumors contain more anti-cancer immune cells. For example, vascular endothelial growth factor (VEGF) related to CD31 is a key mediator of angiogenesis and altered regulation has been implicated in several diseases, including malignancy (13). As shown by Silva et al. in 2022 (14), a close association was revealed between CD31 expression and lower disease-free survival. Bianconi et al. (15) also showed that lower microvessel density assessed by CD31 overexpression was associated with reduced progression-free survival. In another survey by Zhu et al. in 2021 (16), the upregulation of CD31 in tumor cells could predict a higher risk for peritoneal metastasis in colorectal cancer patients. Mohamed et al. in 2017 (17), however, could not indicate the association of CD31 expression with the recurrence rate of colon cancer, but in their study, such a relationship was powerful for other markers including CD105 and VEGF. Sometimes a close correlation was revealed between the different vascular-related biomarkers in malignant tumors. As shown by Deliu et al. (18), the power of correlation between CD31 and CD105 was indicative of vascular invasion in colon cancer. Two important points can be interpreted and extracted from examining the results of the present study and comparing them with other studies. Firstly, the expression level of the investigated marker may be different depending on the disease conditions and background characteristics of the patients, and may play different roles in different societies regarding tumor invasiveness. Secondly, the vascular invasiveness of this tumor can be the result of the interaction and interference of a network of tumoral biomarkers, which sometimes requires the establishment of such an interaction for the occurrence of tumoral invasion. Therefore, what was evident in our study was the high ability to track the expression of the mentioned marker in predicting the vascular status of the tumor and, in fact, the risk of tumor vascular invasion. However, in this study, due to the lack of long-term follow-up of the patients, it was not possible to evaluate the relationship between the expression of this marker and the survival of the patients. On the other hand, it should be kept in mind that the phenomenon of angiogenesis itself can be multifactorial and can be influenced by other much stronger factors. Firstly, different variants of VEGFs and activation of their related pathways have major leading effects on tumor angiogenesis (19-21). Additionally, the determining effects of other factors such as the expression of COX isoforms (COX-2), host-infiltrating cells, and anti-angiogenic factors secreted by endothelial cells have been also demonstrated (22, 23). Some specific proteins related to angiogenesis can be used as target therapy in cancers such as COX-1/2 (24). Biomarker expression and histopathological evaluations are now used to predict tumor behaviors (25). In general, it can be said that in our society, in evaluating the prognostic role of the marker in predicting the microvascular state of the tumor and, therefore, its vascular expansion, considering a set of these factors provides us with more accurate evidence. The limitations of this study were the small sample size in one referral hospital, which may affect the strength of the results. Therefore, repeating the study with a larger sample size in a multi-center is strongly recommended.
5.1. Conclusions
In conclusion, CD31 positivity in patients with colon cancer can effectively predict invasive behavior of the tumor including vascular invasion, lymph node involvement, and high tumor grades. Because the pointed parameters are now identified as the potential markers for cancer poorer prognosis, it is also expected that determining CD31 status in such tumors can also predict disease prognosis that needs to assess in further studies.