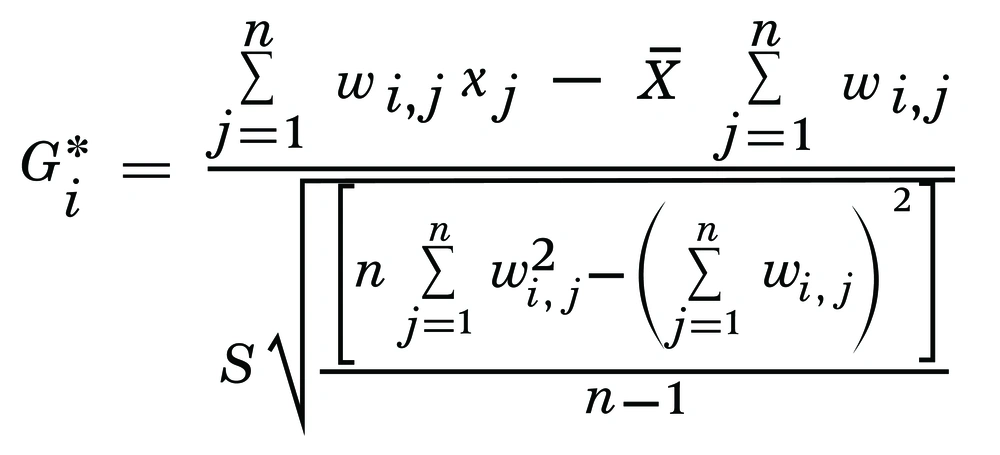1. Background
Breast cancer (BC) is one of the most frequently diagnosed malignant tumors among females globally, comprising 2261419 new cases in 2020. Specifically, it is the fifth leading cause of cancer death in females, as well as a significant cause of cancer death in males throughout the world. According to the GLOBOCAN 2020, the age-standardized incidence rate (ASIR) of BC was 35.8 per 100000 person-years and the number of new cases was 12.9% (1). Surprisingly, the ASIR displayed a slightly upward trend between 1990 and 2019 (2) and it is forecasted that the number of BC new cases will reach over 3 million and the number of deaths to 1 million every year by 2040, which indicate an increase in the burden of disease in the future (3). In Iran, as a developing country located in the Middle East, cancer is the second largest group of chronic non-communicable diseases (4). It was reported that BC accounts for 21.4% of all common cancers in this country (5). From 1990 to 2019, the trend of ASIR from BC has grown dramatically among females in Iran (2). Moreover, the GLOBOCAN study found that the new cases, deaths, and 5-year prevalence due to BC ranking as first cancer among the other cancers in Iran in 2020 (1). Thus, identifying regions with a high risk of BC is extremely important for implementing prevention measures.
It is well reported that the incidence cases and rates of BC vary markedly around the world, such that it is usually greater in high-income countries compared to low-middle income ones (2, 6). This may be due to, in part, differences in the prevalence of risk factors and also variations in the implementation or uptake of screening (7). Moreover, the disparities in BC incidence across the world may be associated with population characteristics, socioeconomic status, and access to healthcare. One of the powerful tools to determine the inequalities in the geographical distribution patterns of diseases is spatial analysis. This method rather than traditional descriptive statistics, can take the co-variation of properties with a geo-space into account, and allow for detecting spatial dependence or autocorrelation in spatial data (8, 9). Geographic information systems (GIS) is a useful method to perform the spatial analysis of disease burden at a finer geography level (10). It can link spatial data with different sources of BC attribute data to develop a big picture for the incidence of BC patterns across geographic areas (11). Using GIS, policymakers can identify significant clusters of both high and low incidence rates in the study area along with potential associated risk factors (12).
Histopathological reports of BC are very crucial to oncologists because they can help to determine the stage of the disease and plan future treatment strategies (13). Pathology reports should be timely, accurate, complete, and usable. A reflection of overall quality in pathology reports is completeness (14). Incomplete case ascertainment may lead to an underestimation of the number of living cancer patients, underestimation of cancer incidence, and prevalence. As a result, it may cause the misinterpretation of trends (15). Therefore, the assessment of the completeness of registry data is very essential.
The statistics suggest that BC is a major concern of general health in Iran (16). Earlier studies conducted in Iran have investigated extensively the burden of BC and it was found that there are variations in BC incidence patterns and distributions in different provinces of the country (17-21). Thus, it should be cautiously interpreted and investigated whether this higher incidence is due to registry issues or as a result of specific exposures. However, limited previous studies performed in Iran have specifically examined the spatial epidemiology of BC to better understand its geographical distribution. Spatial analysis of the incidence of BC can generate a better understanding of variations across provinces and help to identify unmet areas, where the incidence of BC risk is higher or lower. The spatial analysis at the finer geography level may enable the policymakers to tailor prevention strategies to areas, where the BC risk is higher.
2. Objectives
The aim of this study was to determine the geographical hot spots and cold spots of BC incidence in Iran, using spatial cluster analysis. Besides, the completeness of surgery pathology reports related to BC registry data was assessed.
3. Methods
3.1. Study Settings and Data Sources
Iran is a country with a wide latitude and longitude range, diversity of topography, and altitude between 25 and 5671 meters above sea level, with different ethnicities, diverse environmental and climates conditions, and in particular, substantial differences in lifestyles in various socio-economic groups.
Data were collected from the Iranian Cancer Registry System in 2016 based on guidelines issued by the Iranian Ministry of Health defined by ICD10 codes (C50.0 - C50.9) (22). Considering the women population reported by the National Census of Iran Statistical Center in 2016 (23) and the world standard population as a reference, age-standardized rates of cancer incidence were calculated, using the direct standardization method (24). Upon completing data collection, data were entered into Excel spreadsheets, which provided a faster computational environment to be linked to maps in ArcGIS software.
Through a simple random sampling method, 4000 reports were selected from 8940 surgical pathology reports. These reports are recorded from the Integrated Cancer Information Management System (Sima Cancer) prepared by the Ministry of Health, Treatment, and Medical Education. The pathology report is related to the tumor, which includes tumor type, tumor site, tumor size, pathologic T stage, pathologic N stage, and tumor grade. In this study, through the Framework for Specialist Minimum Data Set Development for Specific Cancers in Clinical Cancer Registration, the minimum data set of the pathology report was utilized (25). The study was approved by the Research Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (IR.IUMS.REC.1400.878).
3.2. Statistical Analysis
This study assessed the completeness by reporting the frequency and percentage of data available on clinical pathology reports by age groups (≤ 50, 50 - 60, and > 60 years). The chi-squared test was utilized to examine the difference between the groups. All data were entered and analyzed in STATA 13.0 (StataCorp CLL, College Station, TX). P values less than 0.05 were considered statistically significant.
The most recent updated electronic map of Iran and its provinces was used for this study. An ID field was created in the Excel tables reporting the incidence of BC to link the data tables to the map. Hot spots of BC were identified, using Getis-Ord Gi* (Spatial Statistics) (Figure 1). The term Hot Spot refers to a province that has both a high incidence of disease as well as a high incidence neighboring province. In Figure 1, Getis-Ord Gi* (Spatial Statistics), Xi is the attribute value for feature j and wij is the spatial weight between feature i and j, and n is the total number of features. A cold spot is a province with a low incidence and a neighbor with a similar situation. To determine whether a province is considered a Hot Spot or Cold Spot, a 1.96 SD from the national average was taken into consideration at the level of 0.05%.
4. Results
4.1. Baseline Characteristics of Patients
Data on 4000 surgical pathology reports related to BC patients in 2016 were examined for the current study. Among 4000 patients, the range of age range varied from 20 to 95 years. The mean age of the patients was 51.4 ± 12.5 years. The highest frequency of age was observed in patients younger than 50 years. A total of 3120 (78%) pathology reports were registered in public centers and the remaining reports (22%) were registered in private centers. There was coverage for 3754 (93.8%) patients in one of the health insurances, while 246 (6.2%) patients did not have any type of coverage (Table 1).
| Characteristics | Values |
|---|---|
| Age (y) | 51.4 ± 12.5; 20 - 95 |
| ≤ 50 | 1968 (49.2) |
| 50 - 69 | 1683 (42.1) |
| > 60 | 349 (8.7) |
| Center | |
| Public | 3120 (78) |
| Private | 880 (22) |
| Insurance | |
| Insured | 3754 (93.8) |
| Uninsured | 246 (6.2) |
a Values are expressed as No. (%) or mean ± SD; range.
4.2. Completeness of BC Pathology Reports
Comparing the proportions of tumor type, tumor site, and tumor size, it was found that invasive ductal carcinomas had the highest frequency in patients aged ≤ 50, 50 - 69, and > 60 years with 1303 (66.2%), 1137 (67.6%), and 227 (65%) patients, respectively. The greatest proportion of tumor sites was detected in the upper outer quadrant in the 3 age groups with 1545 (78.5%), 1316 (78.2%), and 268 (76.8%) patients, respectively. Subsequently, the highest frequency was related to evaluable tumor size in the 3 age groups with 641 (32.6%), 620 (36.8%), and 129 (37%) patients. In pathology reports, the tumor type for 613 patients, the tumor site for 173 patients, and the tumor size for 2591 patients were not reported. As well, the relationship between tumor size (P = 0.043) and age was statistically significant. Nevertheless, no significant difference was observed between tumor site (P = 0.332), tumor type (P = 0.178), and age (Table 2).
| Characteristics | Age | Total | P Value b | ||
|---|---|---|---|---|---|
| ≤ 50 | 50 - 69 | > 60 | |||
| Tumor type | 0.178 | ||||
| Ductal carcinoma in situ | 162 (8.2) | 107 (6.4) | 21 (6) | 290 (7.2) | |
| Invasive ductal carcinoma | 1303 (66.2) | 1137 (67.6) | 227 (65) | 2667 (66.7) | |
| Invasive lobular carcinoma | 65 (3.3) | 73 (4.3) | 12 (3.4) | 150 (3.8) | |
| Lobular carcinoma in situ | 100 (5.1) | 69 (4.1) | 20 (5.7) | 189 (4.7) | |
| Others | 37 (1.9) | 42 (2.5) | 12 (3.4) | 91 (2.3) | |
| Not report/ missing | 301 (15.3) | 255 (15.2) | 57 (16.3) | 613 (15.3) | |
| Tumor site | 0.332 | ||||
| Central portion of breast | 279 (14.2) | 228 (13.5) | 55 (15.8) | 562 (14.1) | |
| Lower inner quadrant | 7 (0.4) | 9 (0.5) | 3 (0.9) | 19 (1.1) | |
| Lower outer quadrant | 9 (0.5) | 16 (1) | 3 (0.9) | 28 (0.7) | |
| Nipple | 18 (0.9) | 28 (1.7) | 6 (1.7) | 52 (1.3) | |
| Upper inner quadrant | 18 (0.9) | 17 (1) | 2 (0.6) | 37 (0.9) | |
| Upper outer quadrant | 1545 (78.5) | 1316 (78.2) | 268 (76.8) | 3129 (78.2) | |
| Not report/ missing | 92 (4.7) | 69 (4.1) | 12 (3.4) | 173 (4.3) | |
| Tumor size | 0.043 | ||||
| Can be assessed | 641 (32.6) | 620 (36.8) | 129 (37) | 1390 (34.8) | |
| Cannot be assessed | 11 (0.6) | 8 (0.5) | 0 (0) | 19 (0.5) | |
| Not report/ missing | 1316 (66.8) | 1055 (62.7) | 220 (63) | 2591 (64.8) | |
a Values are expressed as No. (%).
b Chi-squared test.
According to the frequency distribution of pathologic T stage, pathologic N stage, and tumor grade sub-groups, it was observed that the highest frequency was related to the T2 stage (tumor size equal to 2 - 5 cm) in all 3 age groups with 341 (17.3%), 312 (18.5%), and 69 (19.8%) patients. Moreover, the highest frequency of pathologic N stage was related to N0 (histologically, there is no regional lymph node metastasis) in all 3 age groups, accounting for 247 (12.6%), 220 (13.1%), and 42 (12%) patients, respectively. Furthermore, the highest frequency of tumor grade was related to grade 1 with 856 (43.5%), 816 (48.5%), and 176 (50.4%) patients in the 3 age groups, respectively. It should be noted that 2905 patients for the pathologic T stage and 2665 patients for the pathologic N stage were not reported in the pathology reports. On the other hand, the relationship between the tumor grade (P = 0.008) and age was statistically significant. In contrast, there was no statistically significant difference in proportions of the pathologic T stage (P = 0.135) and pathologic N stage (P = 0.820) in age groups (Table 3).
| Characteristics | Age | Total | P Value b | ||
|---|---|---|---|---|---|
| ≤ 50 | 50 - 69 | > 60 | |||
| Pathologic T stage c | 0.135 | ||||
| TX | 11 (0.6) | 8 (0.5) | 0 (0) | 19 (0.5) | |
| T1 | 89 (4.6) | 111 (6.6) | 19 (5.5) | 219 (5.5) | |
| T2 | 341 (17.3) | 312 (18.5) | 69 (19.8) | 722 (18.1) | |
| T3 | 72 (3.7) | 50 (3) | 13 (3.7) | 135 (3.4) | |
| Not report/ missing | 1455 (73.9) | 1202 (71.4) | 248 (71.1) | 2905 (72.6) | |
| Pathologic N stage d | 0.820 | ||||
| NX | 22 (1.1) | 18 (1.1) | 6 (1.7) | 46 (1.2) | |
| N0 | 247 (12.6) | 220 (13.1) | 42 (12) | 509 (12.7) | |
| N1 | 193 (9.8) | 178 (10.6) | 32 (9.2) | 403 (10.1) | |
| N2 | 130 (6.6) | 102 (6.1) | 23 (6.6) | 255 (6.5) | |
| N3 | 58 (2.9) | 58 (3.4) | 6 (1.7) | 122 (3.1) | |
| Not report/ missing | 1318 (67) | 1107 (65.8) | 240 (68.8) | 2665 (66.6) | |
| Tumor grade e | 0.008 | ||||
| Grade 1 | 856 (43.5) | 816 (48.5) | 176 (50.4) | 1848 (46.1) | |
| Grade 2 | 630 (32) | 479 (28.3) | 112 (32.1) | 1218 (30.5) | |
| Grade 3 | 482 (24.5) | 391 (23.3) | 61 (17.5) | 934 (23.4) | |
a Values are expressed as No. (%).
b Chi-squared test.
c TX, primary tumor cannot be assessed; T1, tumor ≤ 20 mm; T2, 20 < tumor ≤ 50 mm; T3, tumor > 50 mm; T4, any size tumor with skin or chest wall involvement
d NX, regional lymph nodes cannot be assessed; N0, no regional lymph node metastasis; N1, metastasis in 1 to 3 regional lymph nodes; N2, metastasis in 4 to 9 regional lymph nodes; N3, metastasis in 10 or more regional lymph nodes or the ipsilateral supraclavicular lymph node
e G1, low grade (score 3 - 5); G2, intermediate grade (score 6 - 7); G3, high grade (score 8 - 9)
4.3. Incidence Rate of BC
A total of 13789 women were diagnosed with BC in Iran in 2016. Among the provinces of the country, the highest and lowest number of new BC cases were observed respectively in Tehran and North Khorasan, and Tehran (56.29) and Sistan and Baluchestan (7.5) had the highest and lowest incidence rates, respectively (Table 4).
| Province | Number | BC Incidence |
|---|---|---|
| East Azerbaijan | 562 | 29.27 |
| West Azerbaijan | 404 | 25.14 |
| Ardabil | 133 | 21.45 |
| Isfahan | 1194 | 47.36 |
| Alborz | 518 | 38.77 |
| Ilam | 112 | 39.30 |
| Bushehr | 129 | 23.77 |
| Tehran | 3712 | 56.29 |
| Chaharmahal and Bakhtiari | 90 | 19.34 |
| South Khorasan | 60 | 15.83 |
| Razavi Khorasan | 1011 | 31.70 |
| North Khorasan | 52 | 12.11 |
| Khuzestan | 859 | 37 |
| Zanjan | 94 | 17.99 |
| Semnan | 105 | 30.37 |
| Sistan and Baluchestan | 103 | 7.5 |
| Fars | 906 | 37.91 |
| Qazvin | 143 | 22.94 |
| Qom | 182 | 28.72 |
| Kurdistan | 122 | 15.44 |
| Kerman | 401 | 25.92 |
| Kermanshah | 303 | 31.42 |
| Kohgiluyeh and Boyer-Ahmad | 58 | 16.49 |
| Golestan | 303 | 32.56 |
| Gilan | 512 | 40.54 |
| Lorestan | 164 | 18.9 |
| Mazandaran | 699 | 42.89 |
| Markazi | 237 | 33.68 |
| Hormozgan | 172 | 19.78 |
| Hamadan | 244 | 28.44 |
| Yazd | 205 | 37.10 |
| Total | 13789 | 34.97 |
4.4. Spatial Analysis
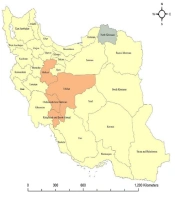
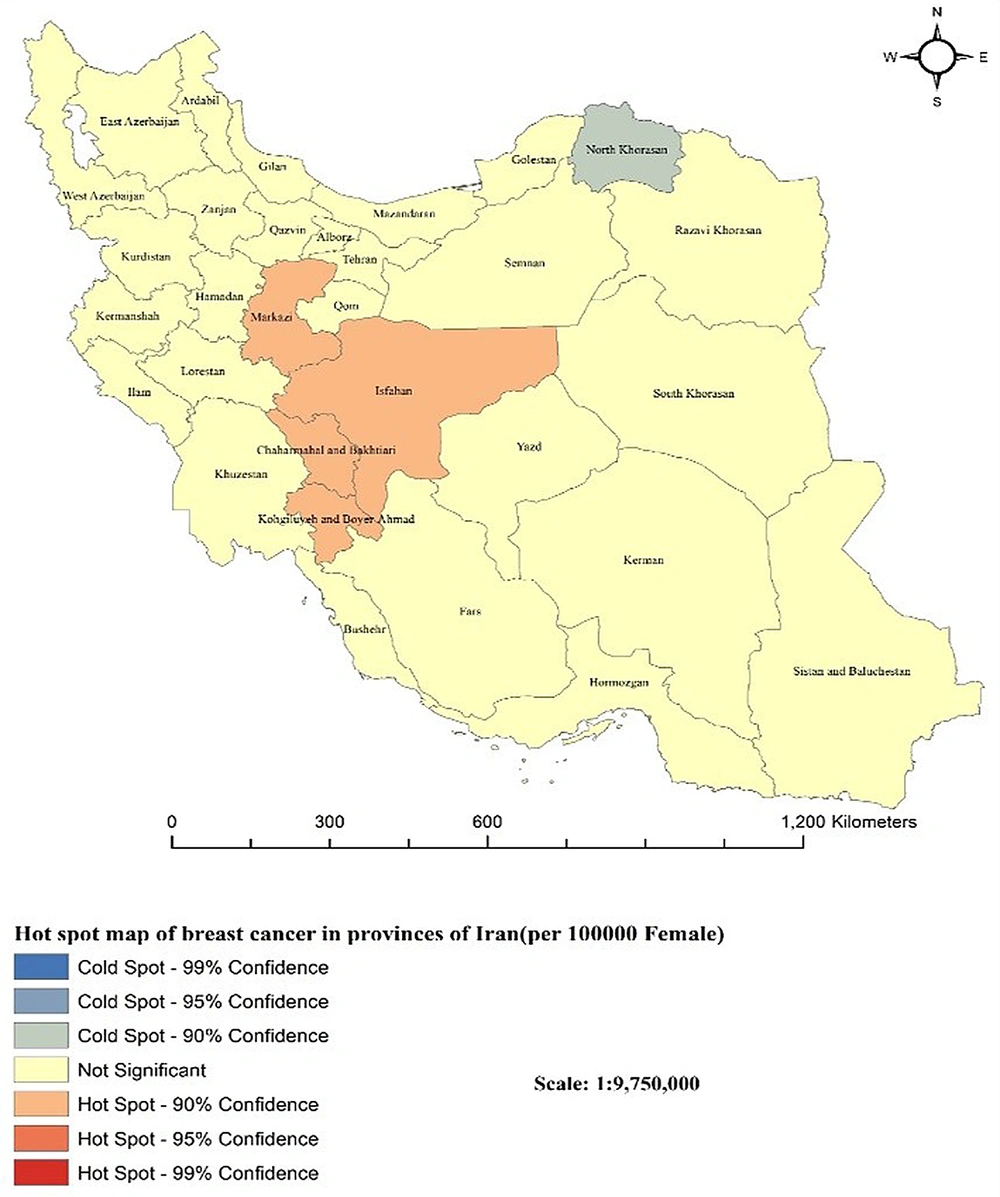
Maps in Figure 2 indicate that outstanding spatial analysis of BC incidence rates (hot spots) was in the central provinces including Isfahan, Markazi, Chaharmahal and Bakhtiari, and Kohgiluyeh and Boyer-Ahmad (P < 0.05). The only clustered province with a low incidence of BC (cold spot) was North Khorasan (P < 0.05). However, the remaining provinces were not regarded as a significant cluster (P > 0.05).
5. Discussion
Previous reports showed that BC is the most frequent cancer in Iranian women and there is an increasing trend for incidence in the country (10, 26, 27). For example, in a cross-sectional study based on Iran’s cancer registry reports, it was found that Isfahan, Yazd, Gilan, and Alborz provinces had the highest incidence rate of BC (21). Factors such as population aging, no full-term pregnancy, late age at first pregnancy, lack of breastfeeding, hormonal pregnancy control, and obesity might be responsible for the trend (28, 29). Investigating the incidence rate due to BC can be important for the government to make policy decisions on allocating resources for early identification and treatment, as well as help to improve the proportion of cancer reported to the registry. Thereby, this article south to describe and discover the population at high risk at Iran's country level.
In the present study, using the data from the Cancer Registry Center report of the health deputy, it was found that Tehran and North Khorasan provinces had the highest and lowest new cases of BC, respectively, in 2016. According to evidence, BC was noted as the most frequent malignancy in Tehran women (27). In large cities like Tehran, due to the increasing social role of women, the social effects of exposure to various risk factors can be more efficacious in the development of BC than in provinces away from Tehran with lower population densities (30). Notably, aging and population growth can be clueing drivers of the high incidence of BC. Besides, demographic transitions can lead to new cases of this cancer being gained differently. Nevertheless, the remarkable improvements in cancer registries and data management should be considered in this city. Roshandel et al., using the database of the Golestan population-based cancer registry (GPCR), detected that the number of new BC cases diagnosed in Golestan had faced a rise from 2004 to 2016 (31). As such, a 93.2% increment in the number of new cases of BC among females is predicted by 2025 in this province (31). This may be partly explained by changes in reproductive characteristics of women, consisting of delayed first birth and decreased parity (32). In another study performed by Fazel et al. conducted on data from GBCR from 2004 to 2013, it was found that most new BC cases occurred among women living in urban areas of Golestan (33). Changes in the lifestyle of women such as delayed first birth, decrease in parity, growing levels of overweight and obesity, and decrease in physical activity may have an important role in the high risk of BC in the province (33). Hence, it is of great importance to evaluate the special requirement and implement optimal BC control strategies according to the cancer profiles in Golestan.
This study supports that among all provinces, Tehran and Sistan and Baluchestan had the highest and lowest incidence rates of BC, respectively. One reason for the high incidence in Tehran may be the availability and highly prevalent use of cancer screening in this province. On the contrary, low use of mammography screening may course a lower BC incidence rate in Sistan and Baluchestan. After Tehran province, Isfahan province has the highest incidence rate of BC; Isfahan province is geographically located in the center of Iran and is ranked as the third most populated province in the country (34). This is a multicultural and industrial zone with various industries. Kazemi et al. by performing a cross-sectional study on 6507 Iranian women living in Isfahan indicated that the ASIR may be due to BC incremented from 22.0/100000 in 2001 to 68.0/100000 in 2013 (35). Elevated BC incidence rate may in some part be due to a more westernized lifestyle, reduced parity and breastfeeding, weight gain, and growth consumption of animal fat (35). Evidence of BC incidence trends in Isfahan yields that 10% of the BC cases in Iran have happened in this province. Using joint point regression analysis, an upward trend in BC incidence was reported from 2001 to 2013 (20). According to an earlier report by the Iranian Ministry of Health in 2014, the ASIR from BC in Isfahan was 42.7 (36). A possible explanation for the difference between our results and the Health Ministry could be owing to the loss of the exclusion of the cases under 20 years old, as well as duplicate data (37). Also, in the study of Mahaki et al., it was shown that the lowest incidence rates of BC in women were from Sistan and Baluchistan province (38). Golestan province, which is located in the north of Iran, has been known as a high-risk area for BC. Recent studies based on the Golestan population-based cancer registry revealed a substantial increase in age-specific incidence rates among young females (39, 40). Comparing the BC ASIR with those of BC around the world, it can be concluded that Golestan is one of the low-risk areas. The low rate can be mainly attributed to epidemiological and demographic characteristics and the cancer registry (41). However, the distinction in diagnosed age of BC, differences in major risk factors, screening strategies, and population size or structures of different regions may be the reasons for the disparities in incidence among different world regions (42).
The major finding of spatial cluster analysis is that the hot spots appeared in Isfahan, Markazi, Chaharmahal and Bakhtiari, and Kohgiluyeh and Boyer-Ahmad in 2016, suggesting that these provinces are known as high-risk areas. While the cold spot is clustered in North Khorasan, which is regarded as a low-risk area. The other provinces were no statistical significance clusters. The hot spots and cold spots confirmed that the BC incidence rate was not randomly distributed. Rahimzadeh et al. reported high levels of geographical heterogeneity in the incidence of BC, using data from the Iranian Ministry of Health data (18). In accordance with our findings, multiple studies conducted in Iran pointed out the ascending trend in BC incidence. The results obtained from a study based on the population-based cancer registry program in Markazi discerned that the ASIR of BC had increased from 27/100000 in 2010 to 45.7/1000 in 2012 (43). In a paper using retrospective data from cancer registry reports in Chaharmahal and Bakhtiari located in Southwest Iran, the authors showed that the trend in BC incidence was rising from 2003 to 2016 (44). Similar prior research investigating the Isfahan province was a hot spot for BC incidence (45). The observed increasing trend in BC incidence in those areas might somehow reflect the noticeable variations in the distribution of risk factors related to shifts in lifestyle and alternating socioeconomic development. More clearly, increasing life expectancy, urbanization, greater exposure to risk factors, delayed childbearing, a higher rate of screening, better cancer registries, and increased surveillance may have an important role in the high incidence of BC. Likewise, in a study, it was shown that Kohgiluyeh and Boyer-Ahmad had the lowest risk of BC incidence, which is inconstant with our results (18). Rafiemanesh et al. in a study using available data from a cancer registry in the North Khorasan province in 5 years (2005 - 2009) declared that one of the most common cancers in this province is BC (46). They demonstrated that BC incidence had an increasing trend until 2008, but then it dropped (46). The low risk of BC in North Khorasan was primarily explained by the potential preventive effects of certain behaviors, the significantly higher average number of pregnancies and parity, and the total years of breastfeeding.
Based on the results of BC pathology reports, it was found that the completeness of tumor type, tumor site, tumor size, pathologic T stage, pathologic N stage, and tumor grade improved with aging. The completeness of cancer data in different age groups varies depending on the type of cancer and the availability of data. Generally, cancer data tends to be more complete for older age groups, as there are more people in these age groups, who have had cancer and are being monitored for it. However, this is not universal and is the opposite in some studies (47, 48). On the other hand, data on cancer are often more complete for certain types of cancers, such as breast and colorectal cancers, which have higher rates of diagnosis and treatment (49, 50). Our results also showed that the tumor grade (100%) and tumor site (95.7%) had the highest, and pathologic T stage (27.4%) and pathologic N stage (33.4%) had the lowest level of completeness among the considered variables. The high completeness may be attributed to the low subjectivity of the interpretation essential. The incomplete reports may be the result of the nature of a pathology-based cancer registry, in which clinically or radiologically diagnosed cases may be missed (51). Thereby, users of cancer registry data should be aware of the potential changes occurring in registry data and even one or two missing cases, which may lead to biases. Improving the completeness and quality of pathology reports is paramount, and may significantly impact the prognosis of the patient (52). In this regard, previous studies have recommended the use of some methods (53, 54). For example, using proforma reporting for pathology reports may grow the completeness rates of the pathology reports up to 96% (55). In synoptic reporting, a prespecified set of items have to be scored before the report and, then, be finalized (54). Generally, for BC, pathology-based cancer registration methods may be proper to complete cancer reports.
There are still some limitations to our study. Firstly, as inherent with all cross-sectional studies, this study could neither establish temporality nor causality of the observed associations with incidence from BC. Secondly, the effect of risk factors was not considered in this study due to the lack of information in the data. At the same time, our research has some strengths. One of the main strengths was the use of nationally representative data. In addition, the use of spatial analytical techniques had advantages over standard statistical methods to determine geographical variations of BC incidence in Iran. This may be of public health significance in the fight against the spread of BC.
5.1. Conclusions
In conclusion, our study has shown that BC incidence rates are highest in certain regions of central Iran. To address this issue, early detection, and effective treatments will be critical in reducing the burden of BC in these areas. However, we also found that standard pathology reports for BC in Iran are incomplete, particularly in terms of tumor size, pathologic T stage, pathologic N stage, and tumor grade. To improve the accuracy and completeness of pathology data, continuous evaluation and complement of data are needed. We hope that our study provides insights that will inform BC control strategies in Iran and that future research will shed light on the factors that contribute to the high incidence rates observed in central Iran.