1. Background
Multiple myeloma (MM) is the second prevalent hematological malignancy accounting for 1.4% of all cancers and 10% of the hematological cancers. It mainly affects males in their 7th-8th decades of life (1). Factors such as health promotion, aging phenomenon, changing in lifestyle, and improved access to the healthcare centers and diagnostic tools have led to increased incidence and diagnostic rate of MM (2). The management of MM is dynamically progressing. Currently, patients are initially managed via chemotherapy, and if the bone marrow gets totally cleared of the cancerous cells, the next step is autologous bone marrow transplantation (ABMT); otherwise, disease relapse occurs (3).
Hematopoietic stem cells (HSCs) reside in the bone marrow niche only, where they are closely interact with stromal cells. Transplantation was performed using bone marrow (BM); however, since the early 2000s, peripheral blood has become the preferred method due to its safety and ease of collection (4).
The process of HSC drawing requires appropriate BM mobilization which is usually stimulated using the granulocyte-colony stimulating factor (G-CSF); however, in case of failure to achieve this goal, successful autologous BM transplantation may not possible (5).
Sympathetic nervous system (SNS) contributes in the regulation of the HSCs regression from the niches in BM. Given that, adrenergic activity affects the stromal cells receptors and mobilizes the cells through molecular signaling. On the other hand, SNS neurons express G-CSF receptors, which limits norepinephrine (NE) reuptake, increasing NE availability and potentiates the sympathetic tone (6).
Desipramine is one of the tricyclic antidepressants acting via the inhibition of NE reuptake; however, it can relatively reduce serotonin reuptake in both central and peripheral nervous system. This characteristic potentially increases sympathetic tone that theoretically in turn might lead to better HSC regression from BM to the peripheral blood (7). However, a preclinical study on mice revealed that desipramine alone was insufficient, but promising outcomes were achieved by its use in combination with G-CSF (8). However, there is limited information available about this medication’s effect on human being.
2. Objectives
The aim of current study was to investigate G-CSF accompanying with desipramine on HSC mobilization among patients suffering from MM.
3. Methods
3.1. Study Population
In this double-centric current randomized clinical trial, a total of 122 patients diagnosed with MM and eligible for autologous bone marrow transplantation were enrolled. The patients were referred to Seyed-o-Shohada Hospital, affiliated with Isfahan University of Medical Sciences, or Ayatollah Taleghani Hospital, affiliated with Shahid Beheshti University of Medical Sciences from January 2018 to March 2019.
The study protocol, designed according to guidelines outlined in Helsinki declaration, was proposed by Ethics Committee of Isfahan University of Medical Sciences and approved under code number IR.MUI.REC.1396.3.693. Besides, the study was registered in the Iranian Registry of Clinical Trials under code number 396693. The study was explained to the patients and/ or their legal guardians, they were reassured about the confidentiality of their personal information and signed written informed consent.
The study inclusion criteria encompassed individual who were over 18 years old, had a documented diagnosis of MM, and were eligible candidates for autologous BM transplantation. Approval of the Transplantation Department of Isfahan University of Medical Sciences was required for their indication for BM transplantation. Additionally, participants needed to have a minimum interval of 7 days between the transplantation and the last session of chemotherapy to be eligible for the study. On the other hand, individuals over 65 years old age, treated with chemotherapeutic regimen of melphalan flufenamide, showing active irresponsive to the chemotherapy for MM, having any chronic medical disease (renal failure, hepatic failure, chronic respiratory diseases and congestive heart failure), and being addicted to opioids were considered unmet criteria. Moreover, reluctance to participation, death during the course of the study, any alteration in the disease approach due to adverse effects, and inability to follow the patients or recruitment of their medical data were defined as the exclusion criteria.
Due to the consensus design of the study, the patients were included to the study through consecutive non-probable selection until achieving the required number of participants. Then, they were randomly assigned to one of the intervention groups using Random Allocation Software.
3.2. Intervention
The first group of participations received treatment with 30 microgram intravenous G-CSF (Abidi Pharmacy, Iran) for a period of five days. This G-CSF treatment was combined with a daily dosage of 100 mg desipramine (Sobhan Pharmacy, Iran), which was initiated within three days before the G-CSF treatment and continued until the last dose of G-CSF.
The latter group were treated with similar dose of G-CSF only.
3.3. Outcomes
The primary outcome of the study was to assess the level of CD34+ cells in order to perform transplantation. The evaluation was done at the end of the interventions and compared between the groups.
The other assessments included white blood cells (WBC) and platelet (PLT) count were also evaluated from a similar blood drawn on the 5th day of the interventions.
Furthermore, the patients’ age, gender, disease stage and frequency of chemotherapy sessions were collected and recorded in the study checklist.
3.4. Statistical Analysis
The obtained data were entered into the Statistical Package for Social Sciences software (SPSS Inc., Chicago, IL, USA) version 23. Categorical data were presented as absolute numbers and percentages, while continuous data were presented as mean and standard deviation. At first, the normality of data distribution was assessed using the Kolmogorov-Smirnov test. For the data with normal distribution, parametric tests were applied; otherwise, non-parametric tests were used. Chi-square test or Fisher’s exact test were used to compare qualitative data and independent t-test and ANOVA were utilized to comparing quantitative data. A significance level of less than 0.05 (P-value < 0.05) was considered statistically significant.
4. Results
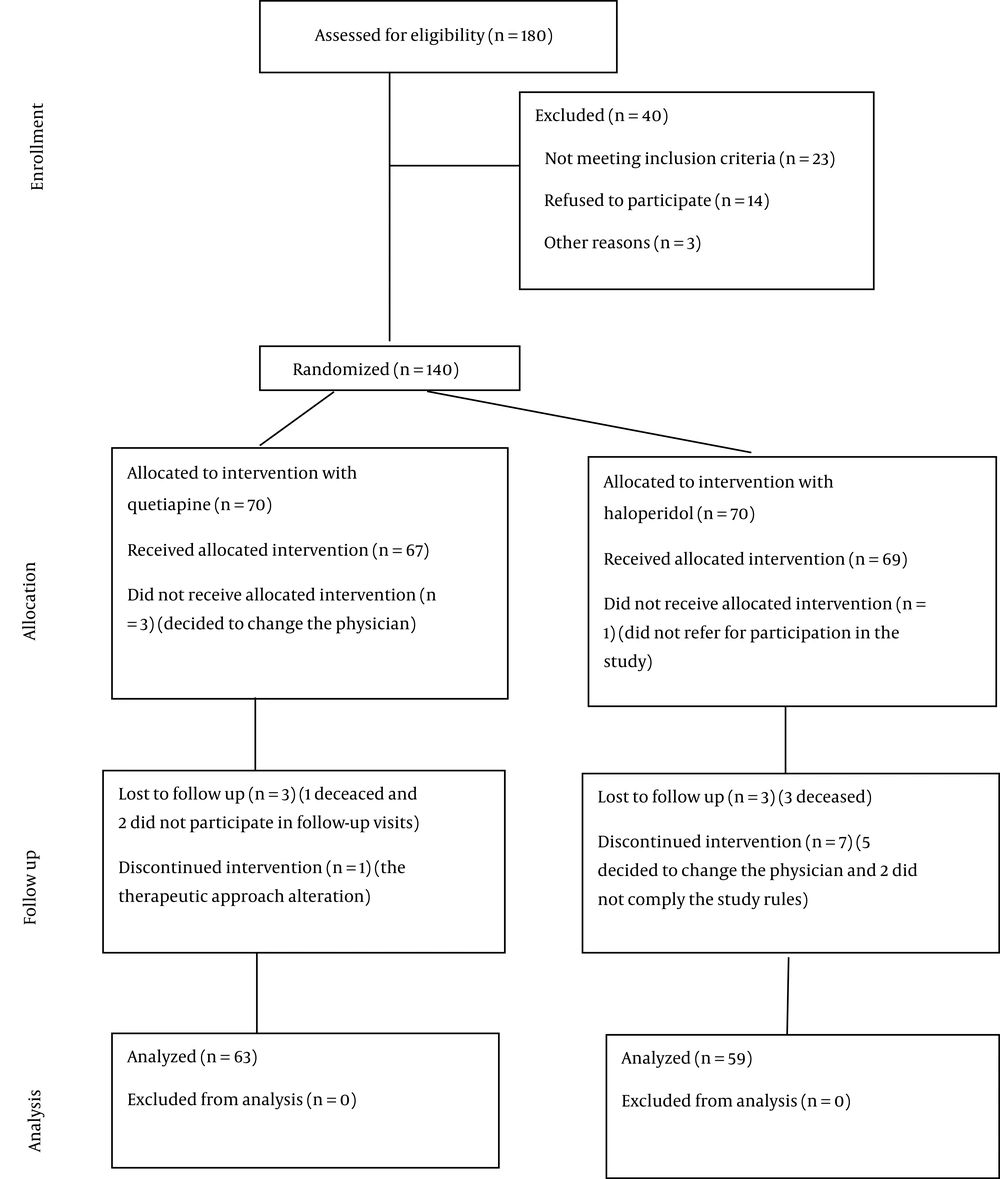
In the current study, the eligibility of 180 patients for participation in the study was evaluated. Amongst them, 40 individuals did not meet the study inclusion criteria and excluded and 18 patients withdrew from the study. Eventually, a total of 122 MM candidate for BMT were included in the investigation. these participants were randomly assigned to two the groups: One group received treatment with G-CSF only (n = 63) and the other group received treatment with G-CSF + desipramine (n = 59) (Figure 1).
The mean age of the studied population was 54.80 ± 8.69 years and the majority of participants were male (60.65%). Table 1 demonstrates the demographic and baseline clinical characteristics of the patients, showing that two groups were similar in terms of age (P-value = 0.486), gender distribution (P-value = 0.160), MM stage (P-value = 0.774), and frequencies chemotherapy sessions (P-value = 0.704).
| Variables | G-CSF Treatment (N = 63) | G-CSF + Desipramine Treatment (N = 59) | P-Value |
|---|---|---|---|
| Demographic characteristics | |||
| Age (y) | 54.27 ± 8.50 | 55.37 ± 8.90 | 0.486 b |
| Gender (male) | 42 (66.7) | 32 (54.2) | 0.160 c |
| Clinical characteristics | |||
| Disease stage | |||
| I | 23 (36.5) | 18 (30.5) | 0.774 c |
| II | 25 (39.7) | 25 (42.4) | |
| III | 15 (23.8) | 16 (27.1) | |
| Frequency of chemotherapy sessions | |||
| 1 | 40 (63.5) | 39 (66.1) | 0.704 d |
| 2 | 23 (36.5) | 19 (32.2) | |
| 3 | 0 (0) | 1 (1.7) | |
a Values are presented as No. (%) or mean ± SD.
b Independent t-test
c Chi-square
d Fisher’s exact test
In general, the patients receiving the combination therapy showed remarkably higher number of CD34+ cells, WBC, and PLT count compared to the G-CSF-treat group (P-value < 0.001) within 5 days after the interventions (Table 2); however, considering the stage of the disease and the frequencies of chemotherapy sessions revealed insignificant differences between the groups (P-value > 0.05) (Table 3).
| Variables | G-CSF Treatment (N = 63) | P-Value | G-CSF + Desipramine Treatment (N = 59) | P-Value |
|---|---|---|---|---|
| CD34 | ||||
| Stage | ||||
| I | 3.11 ± 2.64 | 0.363 a | 4.24 ± 2.79 | 0.837 a |
| II | 2.56 ± 1.35 | 4.64 ± 2.77 | ||
| III | 2.20 ± 1.50 | 4.76 ± 3.27 | ||
| Frequency of chemotherapy sessions | ||||
| 1 | 2.62 ± 1.86 | 0.520 b | 4.85 ± 2.92 | 0.241 b |
| 2 | 2.31 ± 1.29 | 3.96 ± 2.72 | ||
| White blood cells | ||||
| Stage | ||||
| I | 9.52 ± 1.25 | 0.845 a | 11.17 ± 2.33 | 0.481 a |
| II | 9.58 ± 0.77 | 10.60 ± 0.95 | ||
| III | 9.40 ± 0.73 | 10.94 ± 1.06 | ||
| Frequency of chemotherapy sessions | ||||
| 1 | 9.39 ± 0.88 | 0.193 b | 10.95 ± 1.79 | 0.626 b |
| 2 | 9.73 ± 1.03 | 10.74 ± 0.80 | ||
| Platelets | ||||
| Stage | ||||
| I | 90.33 ± 10.39 | 0.289 a | 110.56 ± 20.95 | 0.422 a |
| II | 90.79 ± 10.17 | 100.64 ± 10.99 | ||
| III | 90.20 ± 10.14 | 110.25 ± 10.91 | ||
| Frequency of chemotherapy sessions | ||||
| 1 | 90.53 ± 10.20 | 0.731 b | 110.15 ± 20.51 | 0.694 b |
| 2 | 90.41 ± 10.36 | 100.89 ± 10.81 | ||
a ANOVA.
b Independent t-test.
5. Discussion
The present study aimed to investigate the augmentation therapy with desipramine along with the gold standard regimen of G-CSF to improve peripheral blood HSCs cellularity for BMT.
The study demonstrated that 8 days of daily 100 mg desipramine administration in combination with intravenous G-CSF for 5 days could successfully lead to increased levels of CD34+ cells as the marker of HSCs compared with G-CSF alone. On the other hand, the patients who received the combination therapy exhibited higher WBC and PLT compared to the latter group which is another marker of better outcomes as all the patients experienced bone marrow suppression through the primary chemotherapies. Nevertheless, the impact of the regimens of bone marrow niches was affected neither by the stage of the disease nor by the number of chemotherapy sessions.
The ration by which desipramine has been proposed for bone marrow regression during the process of mobilization refers to its potential effects on the sympathetic nervous system through the inhibition of norepinephrine reuptake. Adrenergic activation of the b3 receptor in bone marrow stromal cells leads to the degradation of the nuclear Sp1 protein, which in turn leads to the repression of C-X-C Motif Chemokine Ligand 12 (CXCL12) transcription, thus enabling stem cell mobilization (9). Accordingly, as the regulation of the β3 adrenergic receptor and the subsequent downregulation of CXCL12 play a crucial role in HSC release; this tricyclic antidepressant has potential to enhance adrenergic activity (10). Surfing the literature represented only one human being study with similar design conducted by Shastri et al. In their study, they intervened with a combination of desipramine and G-CSF in 6 participants and compared them with 13 individuals in the control group who received G-CSF only. They applied daily dose of 100 mg desipramine for a period of 7 days starting4 days before G-CSF initiation. All patients in the intervention group achieved the desired level of CD34+ in a median of 1.5 apheresis session; while, two patients required additional plerixafor. These rates were all superior to the controls who achieved the desired cellularity in a median of 2 apheresis and more than half of them needed additional plerixafor. They continued that this medication was safe and well-tolerated with negligible adversities (11). This study has been derived from the previous ones conducted on mice to assess the potential of desipramine use for bone marrow mobilization. Accordingly, Lucas et al. applied it in combination with G-CSF and represented doubled promoted mobilization among those treated with desipramine and reboxetine, but not desipramine alone (8).
Another notable finding of this study was the elevated levels of WBC and platelets in the desipramine-treated patients representing that not only does desipramine help in the mobilization of the bone marrow; but also contribute to preserving the serum levels of the immune cells derived from myeloid cell lines. In confirmation, Orsini et al. conducted a molecular study aiming at the assessment of inflammatory responses to the shifting of erythropoiesis to myelopoiesis. Therefore, the researchers hued the cells after 9 days of exposure to desipramine and detected elevated levels of myelogenous molecular markers concurrent to the decreased cellular rates of erythroid progenitors. Furthermore, they found that desipramine reinforced the effects of tumor necrosis factor alpha (TNF-α) activity by inhibiting the restoration of erythroid cells during maturation stages (12).
In summary, it should be notified that the current study is only the second trial conducted on human samples; therefore, it probably is a source of bias due to its design and relatively small population; however, it is worth noting that the number of patients are significantly higher than that of the first trial which is a strong aspect of our investigation. Nevertheless, desipramine has some characteristics that warrant further investigations; this medication is easily accessible, cost-effectiveness, and might limit the \amount of G-CSF use, number of apheresis sessions, blood bank resource utilization, and plerixafor requirement. Therefore, conducting further studies are strongly recommended.
5.1. Conclusions
Based on the findings of this study, application of desipramine led to a significant increase in CD34+ cells as the representatives of bone marrow mobilization. Besides, the patients treated with this regimen exhibited higher serum levels of WBC and PLT; however, the response to treatment was not influence by the disease stage and the number of chemotherapy sessions. Despite the limitations of this study, the promising outcomes of this approach suggest the need for further research in this area.