1. Background
Colorectal cancer (CRC) is the third most common cancer in the world and is also among the top five common cancers in Iran (1-3) with more prevalence in men. According to estimates by GLOBOCAN 2020 (1, 2), the number of new CRC cases was over 1.9 million and 900000 annual mortality. CRC causes approximately 9% of all new cancer cases in Iran, with a significant rise (54.1%) in CRC incidence from 2016 to 2025 (4-6). Despite having a high prevalence, detecting colorectal cancer at early stages can be more likely treatable (7).
Cancer registry plays an essential role in any successful cancer control program and population-based cancer registries provide the most valid epidemiological data on cancer (8). In Iran, the first activities of cancer registry were started in the 1950s (9). During early 2000s, the underestimation of incidence rates of pathology-based cancer registration led to the establishment of population-based cancer registries (PBCR) (8, 10). The Iranian National Population-based Cancer Registry (INPCR) covers all 31 provinces and the coverage of population-based cancer data is 100% (8). Comprehensive understanding the extent and severity of tumor to choose the most accurate treatment is vital (11). One of the pivotal factors of cancer care management is the completeness of pathology reports. Ambiguity and incomplete reporting of pathology findings may adversely affect the clinical outcomes (12).
A vast number of pathologists use the checklist suggested by College of American Pathologists (CAP) for colorectal cancer (13). It contains different factors such as specimen type, tumor site, tumor size, macroscopic tumor perforation, tumor type, histologic grade, microscopic tumor extension, margins (proximal, distal and radial), treatment effect (for tumors treated with neoadjuvant therapy), lymphovascular invasion (LVI), perineural invasion, tumor deposits (discontinuous extramural extension), and TNM staging (including the number of resected and involved lymph nodes). At the population level, all these factors can be helpful to conduct epidemiology research and give information for cancer registrations (14, 15). At the individual level, some of the pathology elements are powerful prognostic factors in colorectal cancer including tumor invasion depth, the number of resected and involved lymph, positive/negative of harvested margins, and lymphovascular/perineural invasion (16-20). Since no study has been conducted in this regard in Iran, the aim of this study was to evaluate the completeness of the colorectal cancer pathology reports registered in the population-based cancer registry system in Iran in 2016.
2. Objectives
The aim of this study was to evaluate the completeness of the colorectal cancer pathology reports registered in the population-based cancer registry system in Iran in 2016.
3. Methods
3.1. Data Sources
Iranian National Population-Based Cancer Registry (INPCR) was founded in 2010. Developing a comprehensive national guideline for population-based cancer registries, supporting and supervising the establishment and maintenance of regional cancer registries in provinces, collecting and aggregating regional cancer data to produce and publish national cancer statistics are core objectives of INPCR. Sima-ye-Saratan is a web-based application that was created by INPCR. Its mission is making a secure and facilitate the transmission of data from the university cancer registry secretariat to the INPCR secretariat. There is a unique username/password for each university cancer registry secretariat to access the university dashboard. There are 2 ways to enter data into Sima-ye-Saratan: For individual data, it can be used data entry form and for batch files by import panel.
At the time of this study, Sima-ye-Saratan data was available for the period 2008 - 2018. We requested that the INPCR extracts their pathology records for manual review for colorectal cancer (ICD-10 code ‘C18', ‘C19, ‘C20', and ‘C21') from Sima-ye-Saratan in 2016. Among 5323 pathology reports, 2092 related pathology reports had been reviewed. We extracted data that could also be clinically useful, including the lymphovascular and perineural (not identified, present and cannot be determined), tumor grade (Garde I, II, III, GX), and margin status (involved, not involved, cannot be assessed).
This study was approved by the ethical committee of Iran University of Medical Sciences (IR.IUMS.REC.1400.742).
3.2. Data Collection
The surgical pathology report included various details Extracted such as sex, age, and insurance information. It also included other institutional factors like the date of the report, and reporting laboratory/center (public versus private sector). Additionally, it also contained histopathology characteristics such as tumor grade, tumor type, proximal, distal, and circumferential margin, T (primary tumor), N (lymph nodes), lymphovascular invasion (LVI), and perineural invasion.
3.3. Statistical Analysis
Summary statistics were provided as percentages of categorical variables and mean with standard deviation of continuous variables. A comparison of categorical variables was performed using the Pearson chi-square test. All analyses were performed using SPSS version 20.
4. Results
We assessed 2092 surgical pathology reports that were recorded in Sima-ye-Saratan in 2016. Table 1 describes characteristics of the men and women with colorectal cancer. Men accounted for the largest percentage of sex group with colon and rectal cancer (836 and 335 cases, respectively). Only 105 (11.1%) of the 2092 individuals are uninsured (Table 1) and 69% of colorectal cancer pathology reports were from private sectors.
| Characteristics | Female (%) | Male (%) | Total (%) | P-Value |
|---|---|---|---|---|
| Age | ||||
| ≤ 50 | 205 (22.3) | 262 (22.4) | 467 (22.3) | 0.456 |
| 50 - 69 | 434 (47.1) | 523 (44.7) | 957 (45.7) | |
| ≥ 69 | 282 (30.6) | 386 (33) | 668 (31.9) | |
| Site | ||||
| Colon | 689 (74.8) | 836 (71.4) | 1525 (72.9) | 0.081 |
| Rectal | 232 (25.2) | 335 (28.6) | 567 (27.1) | |
| Center | ||||
| Public | 275 (29.9) | 374 (31.9) | 649 (31) | 0.307 |
| Private | 646 (70.1) | 797 (68.1) | 1443 (69) | |
| Insurance | ||||
| Insured | 870 (94.5) | 1117 (95.4) | 1987 (95) | 0.336 |
| Uninsured | 51 (5.5) | 54 (4.6) | 105 (5) |
Demographic Characteristics by Sex
Table 2 represents the proportion of grade and type of tumor by sex in colon and rectal cancer in which, 78.2% of colon cancer and 80.8% of rectal cancer were Adenocarcinoma. Grade 1 was the most common grade in our study population (Table 2).
| Characteristics | Colon | Rectal | ||||||
|---|---|---|---|---|---|---|---|---|
| Female (%) | Male (%) | Total (%) | P-Value | Female (%) | Male (%) | Total (%) | P-Value | |
| Tumor type | 0.356 | 0.267 | ||||||
| Adenocarcinoma | 544 (79) | 648 (77.5) | 1192 (78.2) | 181 (78) | 277 (82.7) | 458 (80.8) | ||
| Mucinousadenocarcinoma | 60 (8.7) | 65 (7.8) | 125 (8.2) | 18 (7.8) | 25 (7.5) | 43 (7.6) | ||
| Others | 85 (12.3) | 123 (14.7) | 208 (13.6) | 33 (14.2) | 33 (9.9) | 66 (11.6) | ||
| Tumor grade | 0.914 | 0.147 | ||||||
| GX | 63 (9.1) | 71 (8.5) | 134 (8.8) | 18 (7.8) | 42 (12.5) | 60 (10.6) | ||
| G1 | 286 (41.5) | 361 (43.2) | 647 (42.4) | 101 (43.5) | 157 (46.9) | 258 (45.5) | ||
| G2 | 282 (40.9) | 334 (40) | 616 (40.4) | 92 (39.7) | 110 (32.8) | 202 (35.6) | ||
| G3 | 58 (8.4) | 70 (8.4) | 128 (8.4) | 21 (9.1) | 26 (7.8) | 47 (8.3) | ||
Pathologic Characteristics in Colon and Rectal Cancers
4.1. Pathology Reports
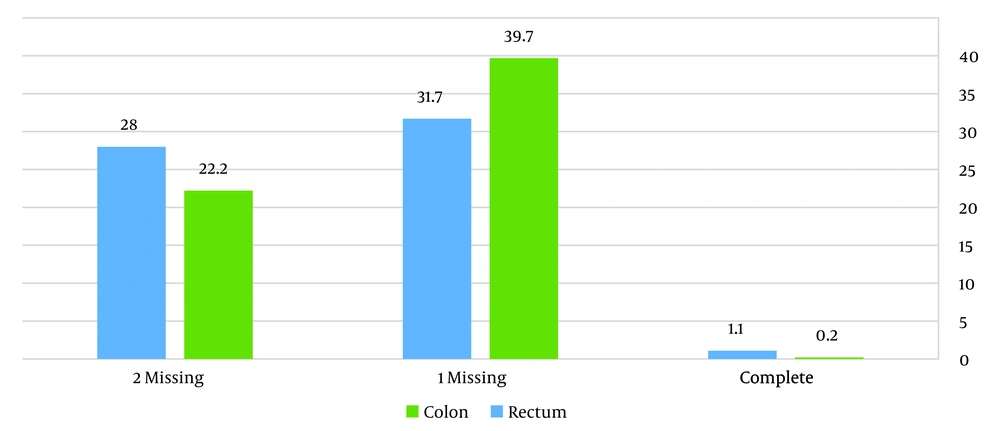
According to Figure 1, which illustrates the overall reporting of pathology factors, only 0.2% and 1.1% of colon and rectal pathology reports were reported completely, respectively. It also can be seen that most of the reports had 1 missing in reporting.
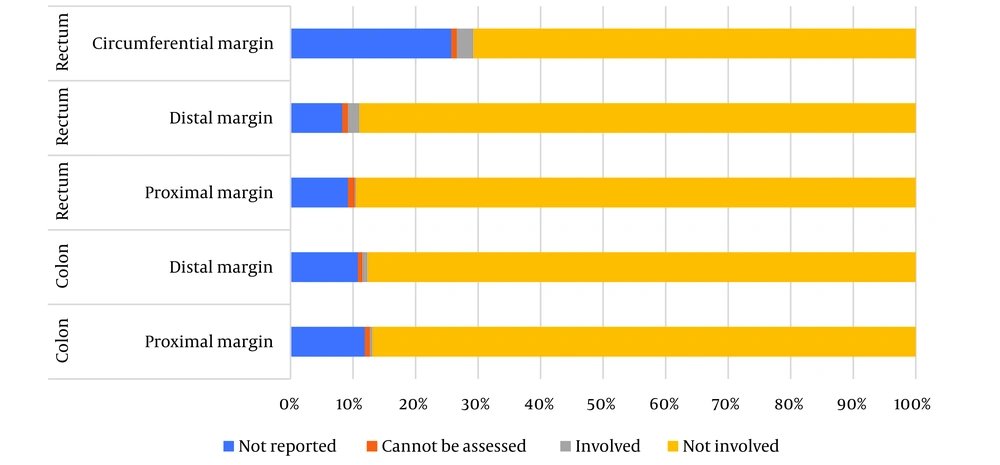
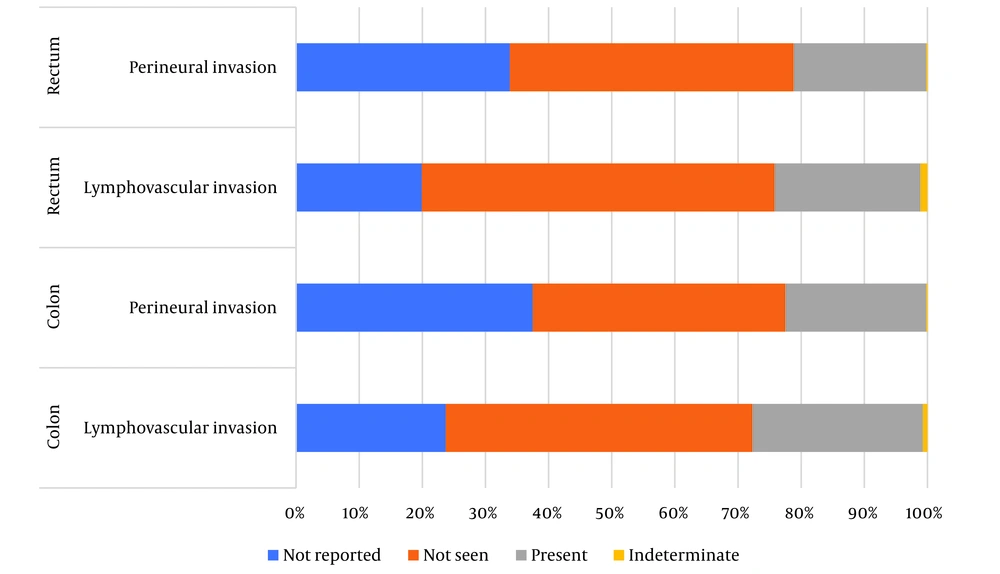
Table 3 shows a summary of pathology factors. In all pathology reports, type (100%) and grade of tumor (100%) were documented. About T-stage, 2.8% of colon cancer and 3.2% of rectal cancer cases were not reported and N-stage was not documented in 7.7% and 8.5%, respectively. Regardless of size of tumor, 92.8% of colon cancer and 93.6% of rectal cancer were documented. In rectal cancer, among the three types of margins that we assessed, the circumferential margin had the most missing (25.7%) (Figure 2). Just over half of LVI (colon cancer: 23.7%, rectal cancer: 19.9%) were missing/not reported (Figure 3).
| Characteristics | Colon | P-Value | Rectal | P-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Female (%) | Male (%) | Total (%) | Female (%) | Male (%) | Total (%) | |||
| T | 0.026 | |||||||
| TX primary tumor cannot be assessed | 1(0.1) | 0 | 1 (0.1) | 0.553 | 0 | 0 | 0 | |
| T0 no evidence of primary tumor | 1 (0.1) | 3 (0.4) | 4 (0.3) | 3 (1.3) | 0 | 3 (0.5) | ||
| Tis carcinoma in situ: invasion of lamina propria | 11 (1.6) | 5 (0.6) | 16 (1) | 4 (1.7) | 4 (1.2) | 8 (1.4) | ||
| T1 tumor invades submucosa | 25 (3.6) | 30 (3.6) | 55 (3.6) | 22 (9.5) | 20 (6) | 42 (7.4) | ||
| T2 tumor invades muscularis propria | 100 (14.5) | 123 (14.7) | 223 (14.6) | 51 (22) | 71 (21.2) | 122 (21.5) | ||
| T3 tumor invades subserosa | 470 (68.2) | 573 (68.5) | 1043 (68.4) | 133 (57.3) | 199 (59.4) | 332 (58.6) | ||
| T4 tumor directly invades other organs | 61 (8.9) | 80 (9.6) | 141 (9.2) | 17 (7.3) | 25 (7.5) | 42 (7.4) | ||
| Not reported/missing | 20 (2.9) | 22 (2.6) | 42 (2.8) | 2 (0.9) | 16 (4.8) | 18 (3.2) | ||
| N | 0.513 | |||||||
| NX regional lymph nodes cannot be assessed | 17 (2.5) | 17 (2) | 34 (2.2) | 0.740 | 10 (4.3) | 13 (3.9) | 23 (4.1) | |
| N0 no regional lymph node metastasis | 394 (57.2) | 455 (54.4) | 849 (55.7) | 125 (53.9) | 198 (59.1) | 323 (57) | ||
| N1 metastasis in 1 to 3 regional lymph nodes | 160 (23.2) | 203 (24.3) | 363 (23.8) | 51 (22%) | 70 (20.9%) | 121 (21.3) | ||
| N2 metastasis in 4 or more regional lymph nodes | 70 (10) | 97 (11.6) | 167 (11) | 21 (9.1) | 31 (9.3) | 52 (9.2) | ||
| Not reported/missing | 48 (7) | 64 (7.7) | 112 (7.3) | 25 (10.8) | 23 (6.9) | 48 (8.5) | ||
Surgical Characteristics (T-Stage and N-Stage)
4.2. Clinicopathological Characteristics
4.2.1. Colon Cancer
Table 3 represents pathology factors that were compared by sex group. Considering T-stage, more than two-thirds of patients (68%) had T3 tumor. Just over half of cases (n = 849) had no regional lymph node metastasis. Table 4 shows that invasion into subserosa or into pericolic or perirectal connective tissues comprised 78.4% of tumor extension.
| Characteristics | Colon | P-Value | Rectal | P-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Female (%) | Male (%) | Total (%) | Female (%) | Male (%) | Total (%) | |||
| Tumor size | 0.821 * | 0.768 | ||||||
| Can be assessed | 634 (92) | 773 (92.5) | 1407 (92.3) | 215 (92.7) | 312 (93.1) | 527 (92.9) | ||
| Cannot be assessed | 3 (0.4) | 5 (0.6) | 8 (0.5) | 1 (0.4) | 3 (0.9) | 4 (0.7) | ||
| Not reported/missing | 52 (7.5) | 58 (6.9) | 110 (7.2) | 16 (6.9) | 20 (6) | 36 (6.3) | ||
| Tumor extension | 0.606 | 0.325 | ||||||
| High grade dysplasia/non-invasive neoplasia | 8 (1.2) | 6 (0.7) | 14 (0.9) | 5 (2.2) | 4 (1.2) | 9 (1.6) | ||
| Invasion into muscularis propria | 82 (11.9) | 113 (13.5) | 195 (12.8) | 44 (19) | 62 (18.5) | 106 (18.7) | ||
| Invasion into submucosa | 26 (3.8) | 26 (3.1) | 52 (3.4) | 17 (7.3) | 19 (5.7) | 36 (6.3) | ||
| Invasion into subserosa or into pericolic or perirectal connective tissues | 538 (78.1) | 658 (78.7) | 1196 (78.4) | 158 (68.1) | 234 (69.9) | 392 (69.1) | ||
| No evidence of primary tumor | 1 (0.1) | 2 (0.2) | 3 (0.2) | 2 (0.9) | 0 | 2 (0.4) | ||
| Not reported/missing | 34 (4.9) | 31 (3.7) | 65 (4.3) | 6 (2.6) | 16 (4.8) | 22 (3.9) | ||
. Surgical Characteristics (Tumor Size and Tumor Extension)
4.2.2. Rectal Cancer
Table 3 presents that 58.6% of rectal cancer cases had T3 tumors. Just under three-fifths of the cases (57%) had metastasis in 1 to 3 regional lymph nodes. In terms of tumor extension, 69.1% of patients with rectal cancer had invasion into the subserosa or pericolic or perirectal connective tissues.
5. Discussion
Comprehensive and complete pathology reports are decisive factors in choosing accurate treatment. Tumor invasion depth, the number of resected and involved lymph, positive/negative of harvested margins, and lymphovascular/perineural invasion are powerful prognostic factors in CRC. Numerous international guidelines have developed a protocol on colorectal cancer pathology to improve its quality.
The purpose of this study was to evaluate whether all the essential parameters were included in the pathology report or not according to the CAP. We focused on analyzing the minimal requirements for surgical specimens. Sima-ye-Saratan provided us with colorectal pathology reports on a large-scale (over 2000 pathology reports). Our study highlights the low rate of overall complete reporting (colon 0.2%, rectal 1.1%) and high rate of incomplete reporting of lymphovascular invasion (colon 23.7%, rectal 19.9%), perineural invasion (colon 37.5%, rectal 33.9%) for colorectal cancer in Iran. Whereas, tumor type and tumor grade were present in all pathology reports.
Gimon et al. conducted a study in Alberta, Canada on 431 pathology reports (21). They analyzed 14 elements that the completeness of reporting tumor extension and histological grade were 62.6% and 82.4%, respectively. While the present study showed a higher percentage of complication (96%, 100%). In another study, Buttner et al. analyzed 5 factors: T stage, N stage, Lymphovascular invasion, histological grade, and tumor perforation (22). In comparison with our study, N stage (87.6%) and histological grade (97.7%) had lower percentages. By contrast, the percentage of the T stage (100%) was at the higher level. Winn et al. conducted an audit on 116 pathology reports in Victoria (23). In this study, T stage (100%), N stage (100%), and histological grade (98%) reported that the percentage of Histological grade was lower compared with our study.
Evaluating surgical margin status should be reported as a core item. Particularly in rectal cancer, circumferential margin involvement is strongly predictive of local recurrence and poor survival (24, 25). However, the evidence of significant margin involvement in colon cancer is not adequate (26, 27). Ihnat et al. conducted a study to assess the impact of CAP on the quality of colorectal cancer pathology reporting (28). The positive involvement of distal and proximal margin in colon cancer with 100% complete reporting was 0% while our study showed 0.8% and 0.4% positive involvement, respectively. In contrast to Ihnat et al., the present study had a low rate of positive involvement of circumferential margin (2.6% versus 15.5%). Perineural invasion in colorectal cancer had negative prognostic implications, notably in stage II disease (29). Our study indicated a higher rate of completeness of perineural invasion in colon cancer compared to Peter Ihna´t et al. study (62.5% versus 35.4%). Similarly, higher rate of presence of perineural invasion was found (22.3% versus 4.1%). In terms of rectal cancer, in our study, the rate of completeness of perineural invasion was lower at 66.1%. However, positive invasion of perineural was higher (20% versus 13.3%).
lymphovascular invasion should be considered as a core item due to having strong prognostic implications for CRC (30). According to several studies, completeness of reporting lymphovascular invasion in CRC was various in different countries; 75.6% (21), 37.2% (22), 88% (23), and in this study 77.3% with 27% and 23% positive invasion in colon and rectal cancer, respectively.
The considerable difference among percentage of reporting pathology factors might partly be explained by the ignorance of some certain features that are important for clinical management. Education and regular assessment with feedback can be the best way to overcome this reason (31). Moreover, having access to training and remote interaction is available for some pathologists. Strengthening laboratory capacity, facilitating implementation and application of a standardized reporting system and connecting pathologists to the most up-to-date information regarding histological techniques, laboratory guidelines and other continuing medical education activities are the results of those partnerships. By contrast, those pathologists who do not have such access are more likely to face problems with producing adequate histopathologic reports due to inadequate equipment and limited access to continued training, and an insufficient number of both pathologists and laboratory support staff (32).
The possible bias of our study is that the evaluation of “completeness of reporting” is restricted by absent or nonreported pathological factors. For instance, when pathologists do not report the data element, it is not obvious whether the feature was evaluated and absent but not reported as “negative” versus a feature that was not evaluated. It could lead us to report an overestimation or lack of reporting in some data factors.
One limitation of this study was that we were unable to report TNM staging of the patient’s disease due to requiring the specification of the presence or absence of distant metastases. This data could be obtained from individual surgeon’s records and requires some time and effort.
Moreover, some pathological factors were in CAP guidelines and were not evaluated in our study because there was not any information about them in the reports such as tumor budding and treatment effect.
To the best of our best knowledge, this is the first study that evaluates the completeness of pathology reports at the national level among CRC patients in Iran. Future studies could be directed towards the impact of adequate pathology reports on clinical decision-making.
5.1. Conclusions
Our results reveal a total incompleteness of colorectal pathology reports. Tumor type and grade had a high rate of completeness (100%) while lymphovascular invasion and perineural invasion which are powerful prognostic factors had a low rate of completeness. We suggest that the preformed checklists for colorectal reporting should be available in software for reporting pathology reports so that missing data will be reduced to a minimum. Sufficient reporting of pathological factors is vital for optimum diagnosis, prediction of prognosis and patient care. Moreover, performing high-quality research also requires complete pathology reports