1. Background
Thrombocytopenia is the primary consequence of allogeneic hematopoietic stem cell transplantation (allo-HSCT), which is associated with high mortality and morbidity (1). Conventional leukemia treatments can damage bone marrow (BM) stromal cells, diminishing the hematopoietic capacity of BM. Also, the systemic inflammation and cytokine release triggered by chemo/radiotherapy impair tissues’ endothelial cells, manifesting as one or multi-organ failures (2). Myeloablative conditioning regimens are another cause of HSCT-related thrombocytopenia and bleeding (3). The preparative regimens interfere with stem cell homing and delay hematopoiesis recovery by impairing BM stromal cells. Common complications after allo-HSCT, such as acute graft-versus-host disease (aGVHD), veno-occlusive disease (VOD), and infection, shorten the platelet (PLT) life span in peripheral blood (4).
In patients undergoing HSCT, a prophylactic blood product transfusion is required to prevent bleeding until engraftment occurs (5). The threshold for PLT and packed red blood cell (RBC) transfusion in these patients are PLT count ≤ 10 × 103/µL and hemoglobin (Hb) level ≤ 7 g/dL, respectively, unless there are other risk factors for bleeding such as sepsis, concurrent use of antibiotics, or other hemostatic abnormalities (6-8). Additionally, the increased PLT transfusion in allo-HSCT patients may manifest a systemic inflammatory response to the onset of organ dysfunction (2).
Some studies indicated that PLT count and PLT transfusion might be directly associated with non-relapse mortality and overall survival (OS) in patients undergoing allo-HSCT (9-12). According to these studies, thrombocytopenia is associated with lower OS and worse prognosis in HSCT complications such as aGVHD, organ failure, and infection in non-bleeding patients (13, 14). Reciprocally, some HSCT complications, such as cytomegalovirus (CMV) infection, directly induce thrombocytopenia and even pancytopenia by inhibiting hematopoiesis (15, 16).
2. Objectives
Regarding the role of conditioning regimens and post-HSCT complications such as aGVHD and VOD in organ dysfunction and the association of these comorbidities with thrombocytopenia, we hypothesized that thrombocytopenia and increased PLT transfusion requirement might be associated with organ dysfunction, aGVHD, and 5-year OS in allo-HSCT patients.
3. Methods
3.1. Patient
This retrospective study was performed based on the data obtained from the clinical records of 184 patients who underwent related allo-HSCT from 2008 to 2018 at the Bone Marrow Transplantation Center of Taleghani Hospital, Tehran, Iran. The study received ethical approval from the Ethics Committee of Shahid Beheshti University of Medical Sciences, and all participants signed the informed consent.
According to their diagnosis, the patients received reduced-intensity conditioning (RIC) (5 days of fludarabine 30 mg/m2 of body surface area, 2 days of CCNU 100 mg/m2, and 1 day of melphalan 140 mg/m2) or myeloablative conditioning (Busulfan 3.2 mg/kg/day for 4 days plus either cyclophosphamide 60 mg/kg/day or fludarabine 30 mg/m2 for 2 days) regimens. The prophylaxis regimen was methotrexate (MTX) and cyclosporine. Since it was common in all the patients, it was not included in the statistical analysis.
The patients' PLT count was measured every morning from admission until discharge by a hematology cell counter (Sysmex, Kobe, Japan). The fully matched donors received granulocyte-colony stimulating factor (G-CSF) 10 mg/kg for 4 days, and their peripheral blood stem cells (PBSCs) were isolated using the apheresis system (Terumo BCT, Lakewood, CO).
Hematopoietic engraftment was monitored following HSCT. The PLT and white blood cell (WBC) engraftment were defined, respectively, as the PLT count ≥ 20 × 103/µL for 3 continuous days without PLT transfusion and WBC count ≥ 1 × 103/µL in 3 consecutive days without a blood product transfusion (17). The endpoint for evaluating the engraftment of WBC and PLT was assumed to be 30 and 50 days after HSCT, separately.
3.2. Red Blood Cell and Platelet Transfusion
The PLT count and hemoglobin value of the HSCT transplanted patients were evaluated every morning during the hospitalization. The PLT transfusions were prescribed when the PLT count reached ≤ 10 × 103/µL or there was clinical bleeding. The single donor (SD) was preferentially chosen to avoid the autoimmune reactions, and random donors (RD) were used when a suitable single donor was unavailable. Packed RBC units were transfused in patients with Hb ≤ 7 g/dL.
3.3. The Hematopoietic Stem Cell Transplantation Outcome
Following transplantation, all patients were monitored for 60 months. Diagnosis and grading of aGVHD were carried out based on established criteria (15). The 5-Year OS was defined as the proportion of patients who are alive 5 years after undergoing autologous stem cell transplantation, regardless of disease status, measured from the date of transplantation to the date of death from any cause or the last follow-up. The 4 types of organ failure are defined as hepatic, cutaneous, intestinal, and pulmonary failure. Liver damage was defined as total bilirubin of > 1.95 mg/dL, which is considered grade 2 hyperbilirubinemia based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events 23 (18) (the normal range for total bilirubin is 0.3 mg/dL to 2.0 mg/dL in our laboratory). Cutaneous manifestations are the most prevalent sign of the disease, which is classically described as erythematous maculopapular/morbilliform eruptions starting on the face, ears, palms, and soles (19). The aGVHD can affect any part of the gastrointestinal tract and cause nonspecific symptoms. For instance, mucositis, which is characterized by oral pain, odynophagia, anorexia, blistering, aphthae, and gingivitis, might be a manifestation of either aGVHD or the result of myeloablative conditioning anatomical sites of inflammation as related to interstitial, vascular, and airway tissue that represent cough, dyspnea, and hypoxemia with or without fever. Chest radiography may show bilateral infiltrates therapy. The diagnostic criteria of respiratory failure are classified based on anatomical site of inflammation, which may involve interstitial, vascular, or airway tissues, or be unclassifiable. Clinical symptoms typically include cough, dyspnea, and hypoxemia, with or without fever. Chest radiography often reveals bilateral infiltrates (20).
3.4. Statistical Analysis
The patients' characteristics are reported as frequency (%) or mean ± standard deviation. The recipient age, diagnosed disease, CMV serostatus, donor-patient gender, conditioning regimen, ABO matching, PLT count on admission and transplantation date, single/random donor PLT transfusion, and packed RBC transfusion were all included in the analysis as variables for predicting 5-year OS and aGVHD. The Logistic regression model was utilized for the univariate and multiple analyses with aGVHD as the outcome of interest. The Cox proportional hazard model was employed for the univariate and multiple analyses when the 5-year OS was the outcome. The associations of the risk factors for both organ failure and organ involvement were evaluated by Fisher's exact test for categorical variables and the Spearman correlation test for continuous variables. The medians of PLT count on both admission and transplantation days were used as a threshold. All analysis was carried out using SAS version 9.4. The significance levels for univariate and multiple analyses were 0.25 and 0.05, respectively. The significance level for the correlation test and Fisher's exact tests was set at 0.05.
4. Results
The descriptive statistics are shown in Table 1. The mean ± SD for the recipient’s age was 32.58 ± 10.85 years. Almost half of the patients (n = 99) were diagnosed with acute myeloid leukemia (AML). Forty-nine patients (26.6%) were CMV seropositive. Busulfan/Cyclophosphamide (Bu/Cy) was the most frequent conditioning regimen among patients (53.8%, n = 99). Seventy-three patients experienced organ failure; 15 cases (8.2%) showed intestinal and 16 cases (8.7%) presented cutaneous failures.
| Variables | Values |
|---|---|
| Recipient age | |
| 15 - 30 | 78 (42.4) |
| 30 - 45 | 70 (38.0) |
| 45 - 65 | 25 (13.6) |
| Missing | 11 (6.0) |
| Diagnosed disease | |
| NHL | 14 (7.6) |
| AML | 99 (53.8) |
| ALL | 46 (25.0) |
| Other | 8 (4.3) |
| HD | 10 (5.4) |
| Missing | 7 (3.8) |
| CMV | |
| Positive | 49 (26.6) |
| Negative | 123 (66.8) |
| Missing | 12 (6.6) |
| Donor-patient gender | |
| F - M | 45 (24.5) |
| M - F | 57 (31.0) |
| F - F | 27 (14.7) |
| M - M | 51 (27.7) |
| Missing | 4 (2.2) |
| Conditioning regimen | |
| Bu/Cy | 99 (53.8) |
| Bu/Fu | 43 (23.4) |
| Bu/Fu/ATG | 16 (8.7) |
| RIC | 26 (14.2) |
| ABO match | |
| Match | 100 (54.3) |
| Mismatch | 75 (40.8) |
| Missing | 9 (4.9) |
| Grade aGVHD | |
| Grade 1 | 9 (9.8) |
| Grade 2 | 13 (14.1) |
| Grade 3 | 16 (17.4) |
| Grade 4 | 32 (34.8) |
| Missing | 22 (23.9) |
| Time of aGVHD | 64.79±19.5 |
| PLT | |
| ≤ 134500 | 60 (31.9) |
| > 134500 | 115 (61.2) |
| Missing | 13 (6.9) |
| Mortality after 100 (d) | 29 (15.7%) |
| Mortality after 200 (d) | 40 (21.7) |
| Mortality after 300 (d) | 42 (22.8) |
| Mortality | 51 (27.7) |
| SD-PLT | 25.40 ± 131.97 |
| RD-PLT | 23.16 ± 36.63 |
| PC | 4.54 ± 7.25 |
| Organ failure | |
| No-organ failure | 111 (60.3) |
| Hepatic | 11 (6.0) |
| Intestine | 15 (8.2) |
| Pulmonary | 0 (0) |
| Cutaneous | 16 (8.7) |
| Hepatic + pulmonary | 1 (0.5) |
| Hepatic + cutaneous | 6 (3.3) |
| Hepatic + intestine | 8 (4.3) |
| Hepatic + intestine + cutaneous | 14 (7.6) |
| Hepatic+ intestine + pulmonary | 0 (0) |
| Hepatic + intestine + cutaneous + pulmonary | 2 (1.1) |
Abbreviations: M, male; F, female; AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; HD, lymphoma; NHL, non-hodgkin lymphoma; HD, hodgkin lymphoma; CMV, cytomegalovirus; Bu, busulfan; Cy, cyclophosphamide; ATG, anti-thymocyte globulin; RIC, reduced intensity conditioning (cyclophosphamide and anti-thymocyte globulin); PC, packed cell; SD-PLT, single donor platelet; RD-PLT, random donor platelet; WBC, white blood cell; PLT, platelet, aGVHD, acute graft-versus-host disease.
a Values are expressed as No. (%) or mean ± SD.
The effects of the risk factors on aGVHD incidence are demonstrated in Table 2. The CMV seropositive patients had 94% greater odds of aGVHD incidence (75% CI: 1.75 - 8.18; P = 0.047). The gender difference between donor and recipient had a significant effect on the aGVHD incidence, with approximately 1.5 times greater odds of aGVHD incidence compared to male-to-male transplants (P < 0.25). The female-to-female transplantations had 41% lower aGVHD incidence odds than the male-to-male transplantations (75% CI: 0.45 - 1.48; P = 0.123). For each unit increase in random donor PLT transfusion, the odds of aGVHD incidence were increased by 2% (75% CI: 1.00 - 1.04; P = 0.228). For each unit increase in packed RBC transfusion, the odds of aGVHD incidence were increased by 36% (75% CI: 1.13 - 1.57; P = 0.041). There was no association between risk factors and incidence of aGVHD in multiple analyses.
| Variables | Univariate | Multiple | ||
|---|---|---|---|---|
| OR (75% CI) | P-Value | AOR (95% CI) | P-Value | |
| Recipient age | 0.850 | - | - | |
| 30 - 45 | 1.05 (0.58 - 1.25) | 0.818 | ||
| 45 - 65 | 0.77 (0.36 - 1.08) | 0.383 | ||
| 15 - 30 (RL) | - | - | ||
| Diagnosed disease | 0.844 | - | - | |
| NHL | 0.88 (0.42 - 2.96) | 0.804 | ||
| AML | 0.75 (0.43 - 2.09) | 0.325 | ||
| ALL | 0.95 (0.53 - 2.73) | 0.891 | ||
| Other | 1.97 (0.81 - 7.67) | 0.266 | ||
| HD (RL) | - | - | ||
| CMV | 0.047 a | 0.22 | ||
| Positive | 1.94 (1.75 - 8.18) | 0.047 | 2.34 (0.59 - 9.19) | 0.22 |
| Negative (RL) | - | - | - | - |
| Donor-patient gender | 0.079 a | 0.194 | ||
| F-M | 1.54 (1.30 - 3.46) | 0.108 | 0.78 (0.204 - 3.01) | 0.72 |
| M-F | 1.51 (1.31 - 3.30) | 0.098 | 1.31 (0.41 - 4.16) | 0.64 |
| F-F | 0.59 (0.45 - 1.48) | 0.123 | 0.20 (0.034 - 1.26) | 0.089 |
| M-M (RL) | - | - | - | - |
| Conditioning regimen | 0.551 | - | - | |
| Bu/Cy | 1.16 (0.24 - 2.52) | 0.652 | ||
| Bu/Fu | 1.16 (0.23 - 2.61) | 0.685 | ||
| Bu/Fu/ATG | 0.49 (0.08 - 1.25) | 0.168 | ||
| RIC (RL) | - | - | ||
| ABO match | 0.290 | - | - | |
| Match | 0.84 (0.50 - 1.02) | 0.290 | ||
| Mismatch (RL) | - | - | ||
| PLT transplant | 0.928 | - | - | |
| ≤ 134500 (RL) | ||||
| > 134500 | 1.75 (0.92 - 3.31) | 0.08 | 2.56 (0.48 - 7.67) | 0.093 |
| SD-PLT | 1.00 (0.99 - 1.00) | 0.522 | ||
| RD-PLT | 1.02 (1.00 - 1.04) | 0.228 a | 1.02 (0.97 - 1.07) | 0.43 |
| PC | 1.36 (1.13 - 1.57) | 0.041 a | 0.77 (0.53 - 1.13) | 0.18 |
Abbreviations: M, male; F, female; AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; HD, lymphoma; NHL, non- hodgkin lymphoma; HD, hodgkin lymphoma; CMV, cytomegalovirus; Bu, busulfan; Cy, cyclophosphamide; ATG, anti-thymocyte globulin; RIC, reduced intensity conditioning (cyclophosphamide and anti-thymocyte globulin); PC, packed cell; SD-PLT, single donor platelet; RD-PLT, random donor platelet; WBC, white blood cell; PLT, Platelet; RL, reference level; AOR, adjusted odds ratio.
a Significant at 0.25.
The impacts of the risk factors on the 5-year OS are illustrated in Table 3. Patients aged between 30 to 45 had a 30% lower mortality risk than patients between 15 - 30 years of age [75% CI: (0.50 - 0.98); P = 0.234]. Compared to patients with Hodgkin’s lymphoma, the patients with non-Hodgkin lymphoma (NHL) and AML had 87% and 56% lower mortality risk, respectively (HD) [75% CI: (0.03 - 0.51); P = 0.083] [75% CI: (0.22 - 0.89); P = 0.180]. Cytomegalovirus -positive patients were 98% more prone to death than CMV-negative ones [75% CI: (1.81 - 8.54); P = 0.041]. Among all gender-incompatible transplantations, male-to-female transplantations had a 2.22 times greater mortality risk compared to male-to-male transplantation [75% CI: (1.49 - 3.31); P = 0.020]. Also, the female-to-female transplantation had a 65% higher mortality risk compared with the male-to-male transplantation [75% CI: (1.00 - 2.73); P = 0.245]. ABO-matched transplantation had more favorable survival than ABO-mismatched transplantation, with a 33% higher death risk than ABO-matched transplantation [75% CI: (0.49 - 0.91); P = 0.142]. The PLT count higher than 134.5 × 103/µL on admission day lowered the mortality risk by 56% [75% CI: (0.4 - 0.78); P = 0.045]. For each unit increase in random donor PLT and packed RBC transfusion, the mortality risk was increased by 1% and [75% CI: (1.00 - 1.01); P = 0.035] 7% [75%CI: (1.05 - 1.09); P < 0.001], respectively.
| Variables | Univariate | Multiple | ||
|---|---|---|---|---|
| HR (75% CI) | P-Value | AHR (95% CI) | P-Value | |
| Recipient age | 0.220 a | |||
| 30 - 45 | 0.70 (0.50 - 0.98) | 0.234 | ||
| 45 - 65 | 0.65 (0.37 - 1.14) | 0.380 | ||
| 15 - 30 (RL) | - | - | ||
| Diagnosed disease | 0.133 a | |||
| NHL | 0.13 (0.03 - 0.51) | 0.083 | ||
| AML | 0.44 (0.22 - 0.89) | 0.180 | ||
| ALL | 0.63 (0.30 - 1.31) | 0.475 | ||
| Other | 1.17 (0.48 - 2.85) | 0.831 | ||
| HD (RL) | - | - | ||
| CMV | 0.041 a | 0.03 b | ||
| Positive | 1.98 (1.81 - 8.54) | 0.041 | 2.50 (1.43 - 6.54) | 0.03 |
| Negative (RL) | - | - | - | - |
| Donor-patient gender | 0.045 a | |||
| F - M | 1.35 (0.84 - 2.18) | 0.455 | ||
| M - F | 2.22 (1.49 - 3.31) | 0.020 a | ||
| F - F | 1.65 (1.00 - 2.73) | 0.245 a | ||
| M – M (RL) | - | - | ||
| Conditioning regimen | 0.796 | |||
| Bu/Cy | 0.59 (0.18 - 1.91) | 0.610 | ||
| Bu/Fu | 0.44 (0.13 - 1.48) | 0.439 | ||
| Bu/Fu/ATG | 0.52 (0.13 - 1.98) | 0.578 | ||
| RIC (RL) | - | - | ||
| ABO match | 0.142 a | 0.02 b | ||
| Match | 0.67 (0.49 - 0.91) | 0.142 | 0.86 (0.43 - 0.96) | 0.02 |
| Mismatch (RL) | - | - | - | - |
| PLT transplant | 0.045 a | |||
| ≤ 134500 (RL) | - | |||
| > 134500 | 0.56 (0.40 - 0.78) | 0.045 | ||
| SD - PLT | 1.00 (0.99 - 1.00) | 0.786 | ||
| RD - PLT | 1.01 (1.00 - 1.01) | 0.035 a | ||
| PC | 1.07 (1.05 - 1.10) | 0.0001 a | ||
| Organ failure | 0.001 a | |||
| Hepatic | 2.80 (1.47 - 5.34) | 0.069 | ||
| Intestine | 0.69 (0.33 - 1.43) | 0.561 | ||
| Pulmonary | NE | |||
| Hepatic+ pulmonary | 0.0 ( - ) | 0.989 | ||
| Hepatic+ cutaneous | 1.14 (0.43 - 3.00) | 0.871 | ||
| Hepatic + intestina | 2.00 (0.96 - 4.17) | 0.273 | ||
| Hepatic+ intestinal+ cutaneous | 2.69 (1.42 - 5.06) | 0.071 | ||
| Hepatic+ intestinal+ pulmonary | NE | - | ||
| Hepatic+ intestinal+ cutaneous+ pulmonary | 2.48 (0.69 - 8.85) | 0.001 | ||
| Cutaneous (RL) | - | - | ||
Abbreviations: aGVHD, acute graft-versus-host disease; AHR, adjusted hazard ratio; RL, reference level; NE, not estimated; M, male; F, female; AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; HD, lymphoma; NHL, non- hodgkin lymphoma; HD, hodgkin lymphoma; CMV, cytomegalovirus; Bu, busulfan; Cy, cyclophosphamide; ATG, anti-thymocyte globulin; RIC, reduced intensity conditioning (cyclophosphamide and anti-thymocyte globulin); PC, packed cell; SD-PLT, single donor platelet; RD-PLT, random donor platelet; WBC, white blood cell; PLT, platelet.
a Significant at 0.25
b P < 0.05 was considered statistically significant.
Similarly, organ failure was associated with the OS. Hepatic failure deteriorates the OS, with 2.8 times greater mortality risk than the patients with cutaneous involvement [75% CI: (1.47-5.34); P = 0.069]. The combination of hepatic, intestinal, and cutaneous failure had a significant effect on OS, as it induced a 2.69 times higher mortality risk compared to cutaneous involvement [(75% CI:(1.42 - 5.06); P = 0.071]. In the multiple analysis, the risk factors, including CMV and ABO matching, were significant.
The association between organ failure risk factors in aGVHD patients and the descriptive statistics for risk factors in each type of organ failure are presented in Table 4. ABO-matched transplants were significantly associated with organ failure (P = 0.027). Two (4.8%) of the matched cases experienced hepatic failure, 4 (9.5%) had intestinal failure, and 3 (7.1%) showed cutaneous involvement. However, PLT count days, receiving single donor PLT (PLTSD) or random donor PLT (PLTRD) units, and number of packed RBC transfusions did not have any significant association with the organ failure (P = 0.441, 0.405, 0.879, 0.322, 0.094).
| Variables | Without organ Failure (%) | Hepatic (%) | Intestinal (%) | Pulmonary (%) | Cutaneous (%) | Value a | P-Value |
|---|---|---|---|---|---|---|---|
| Recipient age | 4.32 | 0.618 | |||||
| 15 - 30 | 28 (73.7) | 4 (10.5) | 5 (13.2) | 0 (0.0) | 1 (2.6) | ||
| 30 - 45 | 20 (60.6) | 4 (12.1) | 6 (18.2) | 0 (0.0) | 3 (9.1) | ||
| 45 - 65 | 6 (66.7) | 0 (0.0) | 3 (33.3) | 0 (0.0) | 0 (0.0) | ||
| Diagnosis | 10.53 | 0.460 | |||||
| NHL | 3 (42.9) | 0 (0) | 2 (28.6) | 0 (0.0) | 2 (28.6) | ||
| AML | 27 (65.9) | 4 (9.8) | 8 (19.5) | 0 (0.0) | 2 (4.9) | ||
| ALL | 16 (69.6) | 4 (17.4) | 3 (13.0) | 0 (0.0) | 0 (0.0) | ||
| Other | 4 (100) | 0 (0) | 0 (0) | 0 (0.0) | 0 (0.0) | ||
| HD | 3 (75) | 0 (0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | ||
| CMV | 1.9 | 0.431 | |||||
| Positive | 6 (85.7) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 0 (0.0) | ||
| Negative | 16 (64.0) | 6 (24) | 3 (12) | 0 (0.0) | 0 (0.0) | ||
| D-P gender | 11.22 | 0.176 | |||||
| F-M | 18 (75) | 0 (0) | 5 (20.8) | 0 (0.0) | 1 (5.6) | ||
| M-F | 19 (63.3) | 4 (13.3) | 6 (20.0) | 0 (0.0) | 1 (3.3) | ||
| F-F | 9 (90) | 0 (0) | 0 (0.0) | 0 (0.0) | 1 (10) | ||
| M-M | 9 (50) | 4 (22.2) | 4 (22.2) | 0 (0.0) | 1 (4.2) | ||
| Conditioning regimen | 5.09 | 0.864 | |||||
| Bu/Cy | 30 (62.5) | 7 (14.6) | 8 (16.7) | 0 (0.0) | 3 (6.3) | ||
| Bu/Fu | 12 (66.7) | 1 (5.6) | 4 (22.2) | 0 (0.0) | 1 (5.6) | ||
| Bu/Fu/ATG | 4 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| RIC | 1 (50.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | ||
| ABO match | 8.56 | 0.027 b | |||||
| Matched | 33 (78.6) | 2 (4.8) | 4 (9.5) | 0 (0.0) | 3 (7.1) | ||
| Mismatched | 21 (53.8) | 6 (15.4) | 11 (28.2) | 0 (0.0) | 1 (2.6) | ||
| PLT transplant | 2.98 | 0.405 | |||||
| > 134500 | 26 (70.3) | 2 (5.4) | 7 (18.9) | 0 (0.0) | 2 (5.4) | ||
| ≤ 134500 | 24 (68.6) | 5 (14.3) | 6 (17.1) | 0 (0.0) | 0 (0.0) | ||
| SD-PLT | - | - | - | - | - | - | 0.879 |
| RD-PLT | - | - | - | - | - | - | 0.322 |
| PC | - | - | - | - | - | - | 0.094 |
Abbreviations: M, male; F, female; AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; HD, lymphoma; NHL, non- hodgkin lymphoma; HD, hodgkin lymphoma; CMV, cytomegalovirus; Bu, busulfan; Cy, cyclophosphamide; ATG, anti-thymocyte globulin; RIC, reduced intensity conditioning (cyclophosphamide and anti-thymocyte globulin); PC, packed cell; SD-PLT, single donor platelet; RD-PLT, random donor platelet; WBC, white blood cell; PLT, platelet.
a Fisher's exact test
b Considered significant.
Table 5 illustrates the association of organ involvement risk factors in patients with no aGVHD and descriptive statistics for risk factors in each type of organ involvement. PLT count days, the number of transfused PLTSD, PLTRD, and packed RBC units did not significantly affect organ involvement (P = 0.65, 0.92, 0.43, 0.64, 0.52). Other risk factors were not significantly associated with organ involvement either.
| Variables | Without Organ Involvement (%) | Infection (%) | Mucositis (%) | Respiratory Involvement (%) | Muscle (%) | Mental (%) | Cardiovascular (%) | Cutaneous Involvement (%) | Value a | P-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Recipient age | 3.34 | 0.85 | ||||||||
| 15 - 30 | 29 (74.4) | 0 (0.0) | 0 (0.0) | 8 (20.5) | 2 (5.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 30 - 45 | 25 (69.4) | 0 (0.0) | 0 (0.0) | 6 (16.7) | 4 (11.1) | 0 (0.0) | 0 (0.0) | 1 (2.8) | ||
| 45 - 65 | 11 (68.8) | 0 (0.0) | 0 (0.0) | 3 (18.8) | 2 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Diagnosis | 5.36 | 0.66 | ||||||||
| NHL | 6 (85.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| AML | 40 (72.7) | 0 (0.0) | 0 (0.0) | 10 (18.2) | 5 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| ALL | 17 (73.9) | 0 (0.0) | 0 (0.0) | 4 (17.4) | 2 (8.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Other | 2 (50) | 0 (0.0) | 0 (0.0) | 2 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| HD | 4 (66.7) | 0 (0.0) | 0 (0.0) | 2 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| CMV | 0.54 | 1.0 | ||||||||
| Positive | 3 (75.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Negative | 38 (70.4) | 0 (0.0) | 0 (0.0) | 10 (18.5) | 6 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| D-P gender | 4.39 | 0.97 | ||||||||
| F-M | 14 (66.7) | 0 (0.0) | 0 (0.0) | 5 (23.8) | 2 (9.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| M-F | 20 (76.9) | 0 (0.0) | 0 (0.0) | 4 (15.4) | 2 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| F-F | 14 (82.4) | 0 (0.0) | 0 (0.0) | 2 (11.8) | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| M-M | 21 (65.6) | 0 (0.0) | 0 (0.0) | 7 (21.9) | 3 (9.4) | 0 (0.0) | 0 (0.0) | 1 (3.1) | ||
| Conditioning regimen | 7.74 | 0.73 | ||||||||
| Bu/Cy | 37 (74) | 0 (0.0) | 0 (0.0) | 8 (16.0 | 4 (8.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) | ||
| Bu/Fu | 18 (75) | 0 (0.0) | 0 (0.0) | 4 (16.7) | 2 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Bu/Fu/ATRIC | 6 (50) | 0 (0.0) | 0 (0.0) | 4 (33.3) | 2 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| RIC | 2 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| ABO match | 2.59 | 0.46 | ||||||||
| Matched | 42 (75.0) | 0 (0.0) | 0 (0.0) | 10 (17.9) | 4 (7.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Mismatched | 23 (63.9) | 0 (0.0) | 0 (0.0) | 8 (22.2) | 4 (11.1) | 0 (0.0) | 0 (0.0) | 1 (2.8) | ||
| PLT transplant | 1.33 | 0.92 | ||||||||
| > 134500 | 32 (76.2) | 0 (0.0) | 0 (0.0) | 6 (14.3) | 3 (7.1) | 0 (0.0) | 0 (0.0) | 1 (2.4) | ||
| ≤ 134500 | 31 (73.8) | 0 (0.0) | 0 (0.0) | 8 (19.0) | 3 (7.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| PLTSD | - | - | - | - | - | - | - | - | - | 0.434 |
| PLTRD | - | - | - | - | - | - | - | - | - | 0.640 |
| PC | - | - | - | - | - | - | - | - | - | 0.529 |
Abbreviations: aGVHD, acute graft-versus-host disease; M, male; F, female; AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; HD, lymphoma; NHL, non- hodgkin lymphoma; HD, hodgkin lymphoma; CMV, cytomegalovirus; Bu, busulfan; Cy, cyclophosphamide; ATG, anti-thymocyte globulin; RIC, reduced intensity conditioning (cyclophosphamide and anti-thymocyte globulin) ;PC, packed cell; SD-PLT, single donor platelet; RD-PLT, random donor platelet; WBC, white blood cell; PLT, platelet.
a Fisher's exact test.
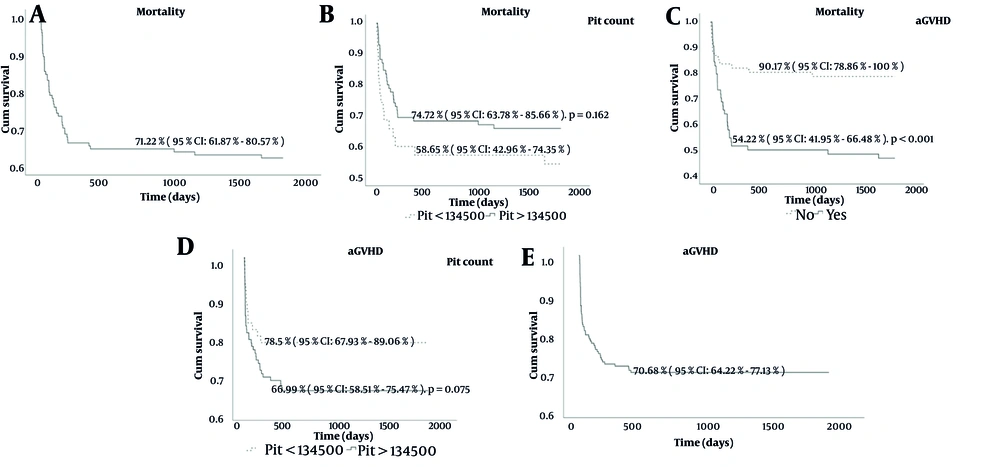
The 5-year OS rate for the cohort was 71.22% (95% CI: 61.87% - 80.57%) (Figure 1A). Platelet count stratification showed that patients with counts > 134,500 had better 5-year OS (74.72%, 95% CI: 63.78% - 85.66%) compared to those with counts < 134,500 (58.65%, 95% CI: 42.96% - 74.35%), though the difference was not statistically significant (P = 0.162) (Figure 1B). Similarly, aGVHD-free survival was higher in patients with platelet counts > 134,500 (78.5%, 95% CI: 67.93% - 89.06%) versus < 134,500 (66.99%, 95% CI: 58.51% - 75.47%) (P = 0.075) (Figure 1C). The presence of acute GVHD had a significant impact, with a 5-year OS of 90.17% (95% CI: 78.86% - 100%) in patients without aGVHD compared to 54.22% (95% CI: 41.95% - 66.48%) in those with aGVHD (P < 0.001) (Figure 1D). Furthermore, aGVHD-free survival for the overall cohort was 70.68% (95% CI: 64.22% - 77.13%) at 5 years (Figure 1E). These findings emphasize the influence of platelet counts and acute GVHD on survival outcomes.
Kaplan-Meier survival curve; A, depicting 5-year overall survival (OS) in the study cohort. The 5-year OS rate is 71.22% (95% CI: 61.87% - 80.57%). Time is measured in days from the date of transplantation. B, Mortality at 5 years by platelet count groups. Kaplan-Meier survival curve showing 5-year OS stratified by platelet count groups. Patients with platelet counts > 134,500 had a 5-year OS of 74.72% (95% CI: 63.78% - 85.66%), while those with platelet counts < 134,500 had a 5-year OS of 58.65% (95% CI: 42.96% - 74.35%), with a P-value of 0.162. C, Kaplan-Meier acute GVHD-free survival (aGFS) curve at 5 years stratified by platelet count groups: PLT < 134,500 with a survival of 66.99% (95% CI: 58.51% - 75.47%), and PLT > 134,500 with a survival of 78.5% (95% CI: 67.93% - 89.06%), P = 0.075. D, Kaplan-Meier survival curve of Mortality at 5 years by Presence of acute graft-versus-host disease (aGVHD). Kaplan-Meier survival curve showing 5-year OS stratified by the presence of aGVHD. Patients without aGVHD had a 5-year OS of 90.17% (95% CI: 78.86% - 100%), while those with aGVHD had a 5-year OS of 54.22% (95% CI: 41.95% - 66.48%), with a significant P-value of < 0.001. E, Kaplan-Meier aGVHD free survival curve (aGFS) of patient at 5 years, showing a survival probability of 70.68% (95% CI: 64.22% - 77.13%).
5. Discussion
Thrombocytopenia is a common complication in patients undergoing HSCT and impacts the HSCT outcome, leading to organ failure, aGVHD, and VOD. The type and stage of malignancy, along with treatment strategy, are the significant factors influencing pre-transplant thrombocytopenia (13). Chemotherapy and radiotherapy cause damage to vascular endothelial cell integrity and release inflammatory cytokines that decrease the survival of platelets in the circulation. Thrombocytopenia persists in HSCT patients due to conditioning regimens (3). Graft-versus-host disease and infection are 2 major concurrent transplant complications leading to morbidity and mortality (21). Immunosuppression with cyclosporine or other calcineurin inhibitors can stimulate endothelial cells to release Von Willebrand factor and express surface adhesion molecules, promoting platelet activation (3).
Moreover, immunosuppressive drugs used in GVHD prophylaxis and management increase infection susceptibility, especially to CMV and Epstein-Barr virus (EBV). Our results showed a higher prevalence of aGVHD in CMV-seropositive patients than in seronegative ones. The 5-year OS is significantly lower in CMV-seropositive patients. Such observation suggests that latent or previous infections might increase the probability of aGVHD and affect the OS rate.
The donor-recipient gender disparity in HSCT can cause allo-reaction and increase the GVHD risk, especially in multiparous female donors (22, 23). Kumar et al. reported that unrelated male donors confer more GVHD in recipients, especially in female recipients, than female sibling donors (24). We found that female-to-male and male-to-female transplantations significantly increased the occurrence of aGVHD, while transplantation decreased the incidence of aGVHD compared to male-to-male transplantation. In addition, male-to-female and female-to-female transplantations increased the OS.
Several studies have emphasized the prognostic significance of the PLT count in patients with GVHD (25-27). We defined PLT count days as cut-off points and investigated the relationship between these thresholds and aGVHD occurrence and OS. We found no significant association between PLT count thresholds at these time points and the incidence of aGVHD. However, the 5-year OS significantly decreased in the patient with a PLT count below the threshold of 134.5 × 103/µL days. Higher platelet counts before transplantation may indicate better BM and immune system function, potentially contributing to improved survival. The infusion of blood products, particularly random donor platelets, can modulate immune responses and increase the risk of aGVHD. Additionally, the presence of residual white blood cells in blood products may play a crucial role in the development of complications. The infusion of packed RBCs can lead to complications due to the excess iron burden in the recipient's body (5, 9).
Post-transplantation pancytopenia increases the risk of bleeding and infection and has been considered a poor prognostic factor in allo-HSCT; thus, patients require multiple transfusions of PLTs and packed RBCs until achieving a stable recovery of hematopoiesis (25, 26). Our results showed that the aGVHD incidence increased, and the 5-year OS decreased in patients who received more packed RBC units. Since RBC transfusion following allo-HSCT is associated with the risk of GVHD development, and worse 5-year OS suggests that the strategies to reduce blood product transfusion may favorably reduce the incidence and severity of GVHD (8, 27, 28). Platelets express minor histocompatibility antigens (MiHAs) and human platelet antigens (HPAs); so, multiple PLT transfusions cause alloreactive reactions (29). Also, platelets directly interact with T-cells through co-stimulatory receptors such as CD40, CD80, CD86, and ICOSL, which might provoke the allo-reactions. Therefore, single donor platelet (SD-PLT) is preferred over single donor platelet (RD-PLT) in HSCT patients. Accordingly, our results illustrated that aGVHD incidence increased in the patients who received RD-PLTs. Besides, RD-PLT transfused patients have a reduced 5-year OS than SD-PLT recipients.
Our findings showed a correlation between organ failure and aGVHD incidence. Similarly, ABO matching status is significantly associated with organ failure in patients with aGVHD. However, no significant relationship was found between risk factors and organ failure in the patients without aGVHD. Gordon et al. indicated that GVHD is associated with endothelial damage, which triggers thromboembolic events and leads to direct or indirect organ dysfunction (2). Reciprocally, systemic inflammation followed by conditioning regimens can damage vascular endothelial cells of organs, resulting in GVHD development. However, we found no correlation between determined PLT thresholds, the number of transfused PLT or packed RBC units, and organ dysfunction.
Managing blood cell production and transfusion therapy involves using hematopoietic growth factors like G-CSF to stimulate blood cell production, medications to reduce bleeding, and leukocyte-reduced blood products to minimize immune reactions (9, 11). Autologous blood transfusions are preferred to reduce infection risks, and strict adherence to transfusion guidelines is crucial to ensure patient safety and improve outcomes (30).
5.1. Conclusions
Pre-transplantation thrombocytopenia, as a major side effect of chemotherapy, can be predictive of HSCT outcome. Systemic inflammation due to aGVHD destroys endothelial cells, increasing the bleeding risk, platelet consumption, and organ dysfunction. Therefore, the blood product requirement increases following HSCT. This study indicates that higher pre-transplant PLT count can be associated with better survival following HSCT. In addition, patients who received more blood products, such as RD-PLT and packed RBC, were at higher risk of aGVHD and had lower survival. Overall, our findings suggested the pre-transplant PLT count as a predictive marker for HSCT outcome.