1. Background
Idiopathic granulomatous mastitis (IGM) is a well-known entity that was described in 1972. It is a rare benign inflammatory disorder that tends to affect women in their reproductive period (1). It can be detected in any part of the breast, occasionally in a bilateral manner. Besides, it may have complications that affect quality of life and even lead to distortion of the breast. In some cases, recurrence and chronicity could happen (1, 2).
IGM has no specific clinical presentation. In almost 75% of the patients, unilateral breast mass is the principal observation (3). Although it is not usual, axillary lymphadenopathy is another symptom of the disease being reported in 15% of the patients (3). In others, skin changes like erythema and nipple retraction were noticed. There is also no pathognomonic sign in ultrasonography, mammography, and magnetic resonance imaging (MRI). Therefore, biopsy and histologic examination are mandatory for accurate diagnosis (4-6).
The pathogenesis of the IGM is not fully understood. Nevertheless, several mechanisms such as abnormal immune response, local stimulant agents, undetected microorganisms, hyperprolactinemia, diabetes mellitus, and oral contraceptives are suggested (7). IGM is only diagnosed when other granulomatous processes like Tuberculosis, sarcoidosis, infective agents, Wegener’s granulomatosis, other collagen vascular diseases, and endocrinopathies are excluded (8-10).
Cystic neutrophilic granulomatosis mastitis (CNGM) is a distinct variant of IGM. It is recognized with suppurative lipogranulomas, consisting of central lipid vacuoles, surrounded by mixed inflammatory cells, epithelioid histiocytes, and Langhans-type giant cells (11). Sometimes, scattered gram-positive bacilli, especially the Coryneabacterium genus, could be present (11).
It should be noted that there is no universal consensus for the treatment of IGM and different strategies like corticosteroid therapy, antibiotics, and surgical removal, and in some studies, close observation were approached (12-14).
2. Objectives
Given that the clinical manifestations of IGM are non-specific and its clinicopathological features are not clear enough, this study aims at investigating the clinicopathological aspects of IGM in patients referred to Shohada-e-Tajrish Hospital, Tehran, Iran.
3. Methods
This retrospective cross-sectional retrospective study was approved by Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1399.297). Patients between 20 to 50 years old who were diagnosed with IGM from 2010 to 2019 and referred to Shohada-e-Tajrish Hospital, Tehran, Iran were enrolled. The information including age, family history of breast cancer, location of IGM (left lower quadrant, left upper quadrant, right lower quadrant, right upper quadrant, and central), clinical feature (pain, erythema, swelling, skin changes, and palpable mass), ultrasound findings (mass-like, hypoechoic, abscess formation, mixed echogenicity, hyper vascularity, and lymphadenopathy), and histopathological features (non-caseating granuloma with mixed inflammatory cells, cystic spaces surrounded by neutrophils, necrosis and sinus tract, and duct ectasia) were collected for all participants.
3.1. Statistical Analysis
The entered data were processed through SPSS software version 22 and the data analyses were performed in two ways descriptive and analytic. The descriptive analysis uses average and standard deviation for quantitative indicators in investigated variables. On the other hand, hierarchical data analysis is performed based on absolute and relative frequency respectively. Likewise, in analytical analysis to determine the relationship between qualitative variables, either Chi-square or Exact tests are performed. P < 0.05 was considered statistically significant.
4. Results
Finally, 60 female patients between 20 to 50 years old and initially diagnosed with IGM were included. The mean age was 34.5 ± 6.73. Overall, 55% of the patients in the left and right upper quadrants were involved. The rate of positive family history of breast cancer was 38.3 in our study.
Also, the main complaints were pain, erythema, swelling, and warmth (51.6%) followed by palpable mass (38.3%). Other objections included in this study were fistula, sinus tract formation, nipple retraction, skin ulceration, and discharge (10.1%).
On the US exam, they were most commonly manifested with a tubular hypoechoic mass (43.3%), while in others, mass-like appearance (21.6%), abscess (15%), and heteroecho lesion were defined. Hypervascularity and axillary lymphadenopathy were infrequently seen.
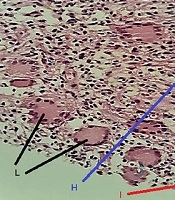
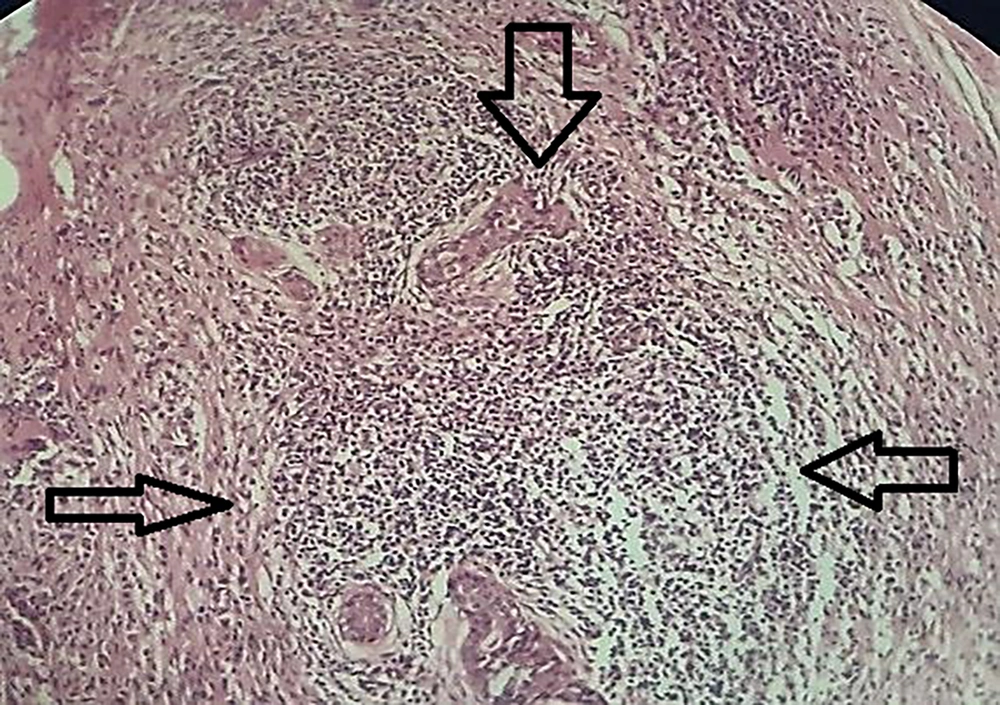
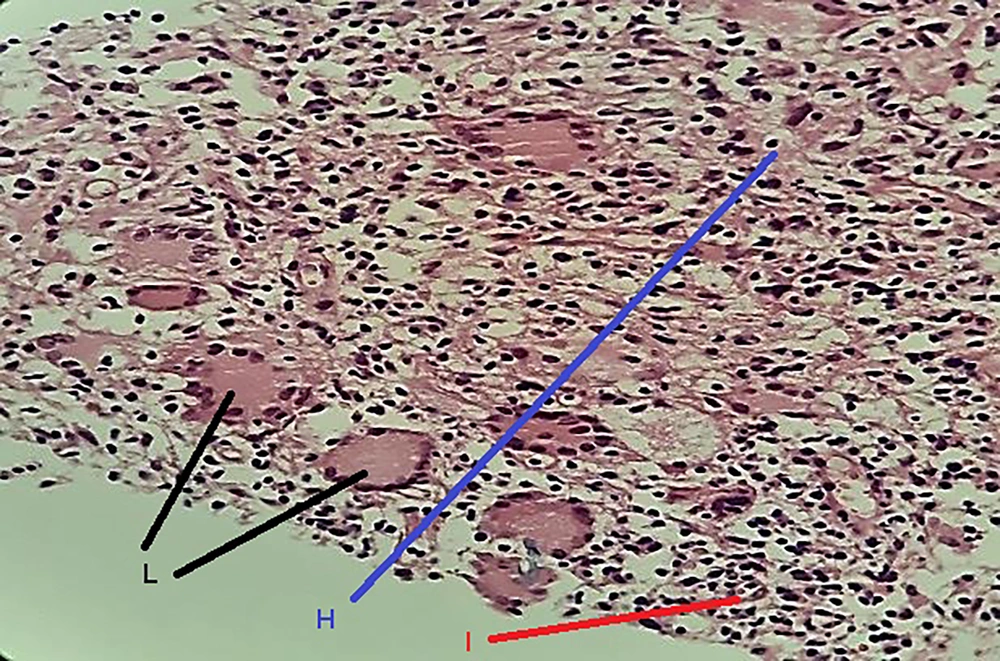
Histologically, non-caseating granulomas associated with mixed inflammatory cells (70%), and cystic space formations surrounded by neutrophil aggregations (15%) composed the main features (Figures 1 and 2). However, the nonspecific manifestations like necrosis, sinus tract formation, and duct ectasia were also described. Microbiology investigations show negative results.
Table 1 displays a summary of the generalized characteristics of the patients.
| Variables | No. (%) of Patients |
|---|---|
| Age, y | |
| ≤35 | 30 (50.0) |
| >35 | 30 (50.50) |
| Overall | 60 (100) |
| Family history | |
| No | 37 (61.7) |
| Yes | 23 (38.3) |
| Overall | 60 (100) |
| Location | |
| LLQ | 4 (6.6) |
| LUQ | 22 (36.6) |
| RLQ | 11 (18.3) |
| RUQ | 14 (23.5) |
| Central | 9 (15.0) |
| Overall | 60 (100) |
| Clinical features | |
| Pain, erythema, swelling | 31 (51.6) |
| Skin changes | 6 (10.1) |
| Palpable mass | 23 (38.3) |
| Overall | 60 (100) |
| Ultrasound findings | |
| Mass-like | 13 (21.6) |
| Hypoechoic | 26 (43.3) |
| Abscess formation | 9 (15.0) |
| Mixed echogenicity | 8 (13.5) |
| Hyper vascularity and lymphadenopathy | 4 (6.6) |
| Overall | 60 (100) |
| Pathology findings | |
| Non-caseating granuloma with mixed inflammatory cells | 42 (70.0) |
| Cystic spaces surrounded by neutrophils | 9 (15.0) |
| Necrosis and sinus tract | 2 (3.4) |
| Duct ectasia | 7 (11.6) |
| Overall | 60 (100) |
Demographic, Clinical, Ultrasound, and Pathologic Features of Participants
We also evaluated the relationship between the age of patients, family history of breast cancer, location of the lesion, clinicopathological findings, and advanced pathologic features like necrosis and sinus tract formation.
As shown in Table 2, it was illustrated that in the patients younger than 35 years old with a family history of malignancy, that presented with a mixed-echo centrally located mass, advanced pathologic features such as necrosis and sinus tract formation were identified concomitantly. However, the k2 test did not support this hypothesis.
| Variables | Pathologic Findings | P-Value | OR | |
|---|---|---|---|---|
| Granuloma with Mixed Inflammatory Cells, Cystic Neutrophilic, Abscess | Necrosis, Sinus Tract | |||
| Age | 0.51 | 1.52 | ||
| <35 | 25 (83.3) | 5 (16.7) | ||
| >35 | 23 (76.7) | 7 (23.3) | ||
| Overall | 48 (80) | 12 (20) | ||
| Family history | 0.35 | 1.82 | ||
| No | 31 (38.3) | 6 (61.7) | ||
| Yes | 17 (73.9) | 6 (26.1) | ||
| Overall | 48 (80) | 12 (20) | ||
| Location | 0.61 b | - | ||
| RUQ | 12 (80) | 3 (20) | ||
| RLL | 7 (77.8) | 2 (22.2) | ||
| LUQ | 14 (77.8) | 4 (22.2) | ||
| LLQ | 8 (100) | 0 (0) | ||
| Central | 7 (70) | 3 (30) | ||
| Overall | 48 (80) | 12 (20) | ||
| Clinical features | 0.49 b | - | ||
| Pain, erythema, swelling | 27 (84.4) | 5 (15.6) | ||
| Palpable mass | 15 (78.9) | 4 (21.1) | ||
| Skin changes | 6 (66.7) | 3 (33.3) | ||
| Overall | 48 (80) | 12 (20) | ||
| Radiologic findings | 0.91 b | - | ||
| Hypoechoic | 27 (84.4) | 5 (15.6) | ||
| Mass like | 15 (78.9) | 4 (21.1) | ||
| Mixed echogenicity | 6 (66.7) | 3 (33.3) | ||
| Overall | 48 (80) | 12 (20) | ||
Relationship Between Pathology Findings of Patients with Their Background Variables a
5. Discussion
IGM is an uncommon benign inflammatory disorder that tends to occur in childbearing women. It usually represents a palpable mass in women, who have a history of pregnancy, breastfeeding, or hormonal therapy (15-17). In this study, the mean age was 34.5 ± 6.73, and the age range of patients (20 - 50 years) complied with related articles (14, 18). In some exceptions, postmenopausal women or men may be affected (14, 19).
According to our analytical results, 38.3% of the patients had a family history of breast cancer. Similarly, in a recent study in Turkey, Yaprak Bayrak showed that a family history of breast cancer and associated disorders was observed in patients and they may have been considered as risk factors; however, that could happen very rarely (20).
In the current study, the most common location of the lesion was the upper outer quadrant (UOQ) with the predominance of the left side and the patients commonly presented with palpable mass and inflammatory manifestations including pain, erythema, and swelling. These results were coordinated with other studies. For example, in Yazdanian’s study, palpable mass and erythema were the most common symptoms (21). Shojaee et al. also stated that all of the patients had palpable masses, and 55.2% presented with erythema and inflammation (22).
In the study of Pala et al. in 2022 in Turkey, the major findings were palpable mass, abscess, tenderness, and skin changes (23).
However, GLM has a wide and diverse spectrum of possible local manifestations. In various studies, unilateral or bilateral painful hard masses, abscess formation, and breast skin changes are reported. Fistula and Sinus tract formation, nipple retraction, and Pea’ de orange appearance are the other occasional findings. They may persist for a few months or relapse (24).
Ultrasound (US) is usually the first line modality and it could be helpful to differentiate between the other possible etiologies, although it has no pathognomonic sign (25). In our case series, the findings were more compatible with a hypoechoic mass, tumor-like lesion, or abscess formation. With less frequency, mixed echo or acoustic shadowing due to increased vascularity were reported. Enlarged axillary lymph nodes were infrequently seen.
These observations are aligned with other reports. In a study by Gautier et al. in 2013, the asymmetrical parenchymal density of the breast was the most common mammographic feature (26), whereas, in the US, a hypoechoic mass with tubular extension and striated echotexture was observed (27, 28).
Regarding various and non-specific clinicopathological features, a confirmatory histopathological diagnosis is mandatory. Typically, a lobulocentric granulomatous inflammation accompanied by multinucleated giant cells and neutrophilic aggregations were common features in this study. Additionally, it was illustrated that the presence of necrosis, fibrosis, sinus tract formation, and duct ectasia in a minority of cases could be related to some background variables such as age, family history of breast cancer, location of the lesion, and radiology findings. However, further analytical testing did not support this.
In a literature review conducted by Anousha in 2022, a total of 192 articles were retrieved to review the characteristics of IGM (27). They concluded that further laboratory testing including culture and molecular testing is necessary to exclude other differential diagnoses (27). Furthermore, they announced that the presence of some histopathologic features including cellular atypia, caseous necrosis, distinct eosinophilic infiltration, and absence of granulomatous inflammation is atypical in IGM and necessitates a careful search for alternative diagnoses (27).
5.1. Conclusions
Although IGM has a generally good prognosis, it can make a challenge in differential diagnosis with other entities. As we declared in our study results along with similar articles, IGM has various characteristics in the clinical context and nonspecific radiologic manifestations necessitating teamwork and collaboration of clinicians, radiologists, and pathologists to avoid an erroneous diagnosis and unnecessary surgeries.