1. Background
Natural killer (NK) cells play a vital role in the innate immune system with the capability to eradicate diverse tumor cells without a need for prior antigen sensitization (1, 2). Indeed, these cells express a repertoire of inhibitory and activating receptors, enabling them to recognize malignant transformation (3). Due to their unique characteristics, during the last decades several attempts have been made to use NK cells in treating leukemia, melanoma, renal cell carcinoma, etc. (4). For this purpose, NK cells have been isolated from different sources including peripheral blood (PB), umbilical cord blood (CB), etc. (4, 5). However, since NK cells constitute approximately 10% and 20% of the lymphocyte’s population in PB and CB, the number of NK cells isolated from these sources is limited and may not be sufficient for multiple infusions (6, 7). On the contrary, NK cells have only an approximate half-life of 7 days (8), thus multiple infusions are necessary to provide long-lasting therapeutic results.
To circumvent the above-mentioned hurdles in NK cell numbers and availability, several preclinical and clinical studies have attempted to exploit hematopoietic stem cell (HSC)-derived NK cells for adoptive immunotherapy (9). For this end, previous studies have explored the anti-tumor activities of NK cells derived from various HSC sources, including bone marrow, fetal liver, and spleen (9). There is strong evidence showing that these HSCs-derived NK cell products are safe, and produce anti-tumor effects; however, consistent tumoricidal effects are yet to be seen (9). Indeed, although promising results have been obtained, but as different sources of HSCs have distinct characteristics, thus NK cells derived from different HSC sources might be different in their anti-tumor potency. Accordingly, research in this field is still ongoing to identify more clinically efficient MSCs to isolate NK cells as well as to develop potential strategies to produce functionally active higher numbers of NK cells for cancer immunotherapy (10).
2. Objectives
In this study, we adopted a protocol for the development and expansion of CD56+ NK cells from cord blood hematopoietic stem cells (CB-HSCs). Numerous studies have utilized either CD34+ HSCs from BM or CB for the differentiation of NK cells. However, in most cases, the culture systems are not applicable for large-scale use, and still some protocols reported are unable to produce high NK cell numbers.
3. Methods
3.1. Cell Lines and Hematopoietic Stem Cells Isolation
Cell lines including K562 and MCF-7 were obtained from Iranian Pasteur Institute and cultured in 10% fetal bovine serum supplemented RPMI1640 containing 1% penicillin/streptomycin, and incubated at 37°C, 5% CO2 with 95% humidity. Samples of CB were obtained from the mothers who underwent normal full-term delivery at the time of birth. A written informed consent letter has been taken under the approval of the ethical committee (IR.TUMS.HORCSCT.REC.1399.022). For HSCs isolation, CB samples were stored at room temperature and then were processed within the next 24 h. In the first step, the collected samples were used to isolate mononuclear cells (MNC) using Ficoll-Hypaque (GE Healthcare, Uppsala, Sweden) density gradient centrifugation. Then, CD34+ HSCs were isolated from MNCs using a commercial immunomagnetic bead separation kit (Miltenyi Biotech, Bergisch Gladbach, Germany) based on the instructions provided by the manufacturer. Afterward, the CD34+ cells were analyzed by flow cytometry for the expression of CD34 and CD45.
3.2. NK Cell Differentiation from Umbilical Cord Blood Stem Cells
For NK cell differentiation, the freshly isolated CD34+ HSCs from CB were cultured for 21 days (3 weeks) in the differentiation media as described elsewhere (11). In detail, CD34+ CB cells were cultured at a NOTCH ligand-coated plate in the NK cell differentiation media containing the cytokine cocktail of 25 ng/mL IL-7, 25 ng/mL stem cell factor (SCF), 25 ng/mL TPO, and 50 ng/mL IL-15 (Table 1). The cells were cultured in the differentiation media containing cytokines under incubation at 37°C, 5% CO2 in a humidified atmosphere, and were replenished with new fresh media every 3 days. After 21 days of incubation under the differentiation condition, the cells were washed and further incubated for expansion (7 days) with the addition of IL-15 and IL-2 cytokines. Finally, the differentiated NK cells were analyzed for the phenotypic features as described in the following section.
| Variables | Concentration (ng/mL) |
|---|---|
| Differentiation phase | |
| SCF | 25 |
| TPO | 25 |
| rhIL-7 | 25 |
| rhIL-15 | 50 |
| Expansion phase | |
| IL-15 | 50 ng |
| IL-2 | 500 IU |
3.3. Phenotyping of CB-Derived NK Cells
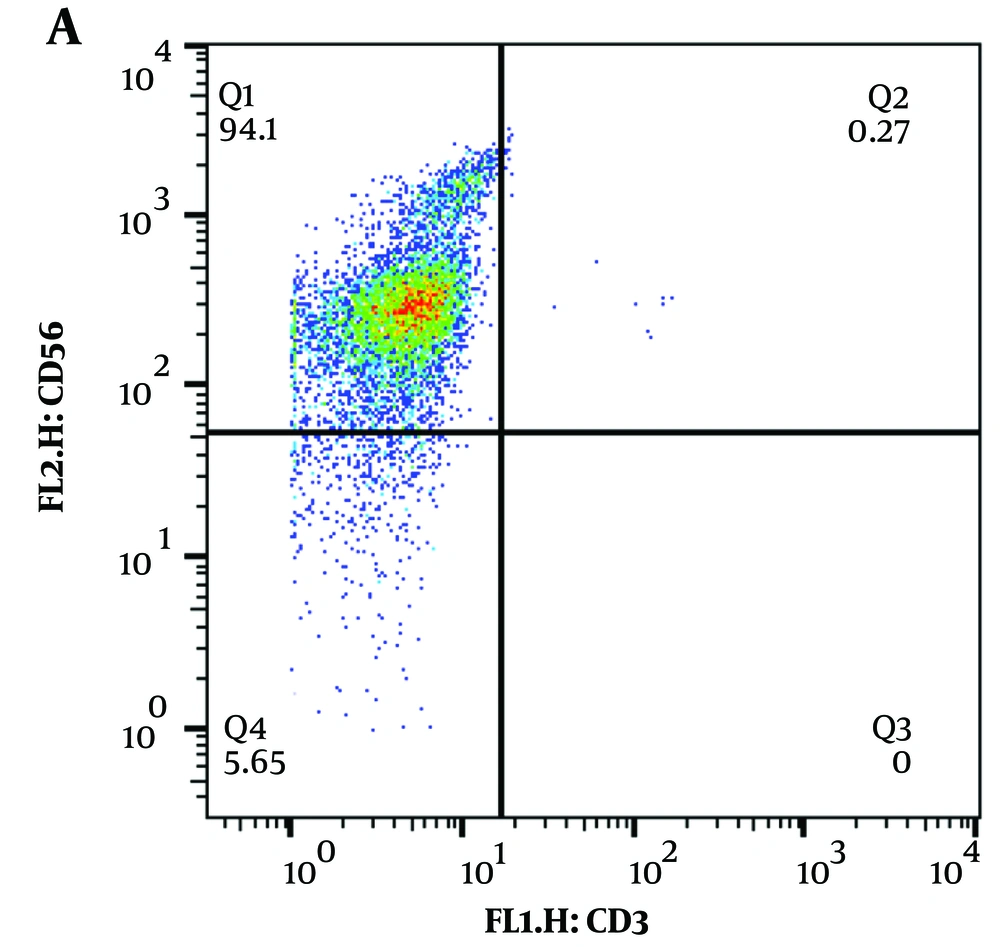
Phenotypic analysis was done to characterize the NK cells derived from CB-HSCs. For this purpose, the following monoclonal antibodies were employed: Anti-CD3 (BioLegend), anti-CD16 (BioLegend), anti-CD56 (BioLegend), anti-NKp30 (BioLegend), anti-NKp44 (BioLegend), and anti-NKp46 (BioLegend). The cells were stained by the above-mentioned fluorochrome-conjugated mAbs under incubation at 4°C for 30 min with, washed and then were resuspended in isotone diluent (Beckman Coulter) and analyzed using the Coulter FC500 flow cytometer (Beckman Coulter). Of note, the numbers and purity of the NK cells were tested by forward and side scatters and by gating on CD45+ cells, respectively. The obtained NK cells were tested for the expression of activating receptors after differentiation (day 21) and expanding period (day 28). The CB samples were taken from 9 donors and at least a set of triplicate experiments were done for each donor.
3.4. NK Cell-Mediated Cytotoxicity Assay
The tumor lytic potential of the CB-HSC-derived-NK cells against K562 and MCF-7 cell lines was measured by XTT (Sigma) assay as described elsewhere (12, 13). In this regard, 0.25 mg/mL of XTT solution was prepared in PBS. A 0.05 mg/mL solution of (2,3)-dimethoxy-5-methyl- (1,4)-benzoquinone (coenzyme Q; Sigma) was also prepared. For each experiment, the fresh stock solutions of XTT were prepared containing 1 mL XTT with 8 μL coenzyme Q. A. Afterward, for cytotoxic tests, 1 × 104 cells/well of target cells (K562 cells and MCF-7 cells) were transferred into U-bottom 96-well culture plates and coincubated with effector cells (NK cells) with different effector to target cell ratios (20:1, 10:1, 5:1, and 1:1). Control groups of effector and target cells were also included. The cultures were incubated for 4 h and then centrifuged for 10 min by 3000 rpm. The supernatants were discarded and replaced by 150 μL of XTT stock solution containing coenzyme Q. The plates were shaken for 1 h at 37°C and then were centrifuged 10 min at 3000 rpm. Finally, 100 μL supernatant of each well was collected into another 96-well plate to measure the absorbance at 490 nm (Universal Microplate Reader, ELx 800 UV). The cytotoxic percentage was calculated using the following formula:
3.5. Apoptosis Assay
Natural killer cell-mediated apoptosis of tumor cells was tested using Annexin V-FITC/PI kit (BD, Bioscience, 556,547). The effector: Target ratio of 10: 1 was selected and the cells were incubated for 6 hours. Afterward, the flow-cytometry analysis was done to determine the percentage of apoptotic cells.
3.6. Chemokine Release Assay
The TNF-α and IFN-γ production by tumor cell-experienced NK cells in their supernatant was measured by ELISA (Pierce Endogen, Rockford, IL, USA). The absorbance of 450 nm by ELISA reader (Titertek, Huntsville, Alabama, USA) was considered as the concentration results.
3.7. Statistical Analysis
Data are presented as the mean standard deviation. The SPSS 16.0 software package was used to analyze the data. The student T-test was employed for comparing quantitative data. P-value < 0.05 was considered as significant.
4. Results
4.1. Isolation of HSC from Cord Blood
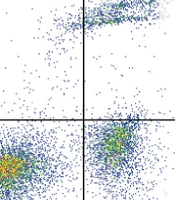
As previously stated, freshly collected cord blood, obtained within the last 48 hours, was employed for the isolation of CD34+ + HSCs. For this purpose, MNCs were isolated from cord blood cells by density gradient centrifugation and then a Miltenyi MACs kit was employed to isolate the CD34+ HSCs. The obtained HSCs were tested for the expression of CD45 and CD34, and as shown in Figure 1, the isolated HSCs were highly pure (CD34+ and CD45-).
4.2. CD34+CB-HSCs Differentiate to NK Cells
The isolated HSCs from cord blood samples were then used for the NK cell differentiation. The cells were cultured under the conditions summarized in Table 1.
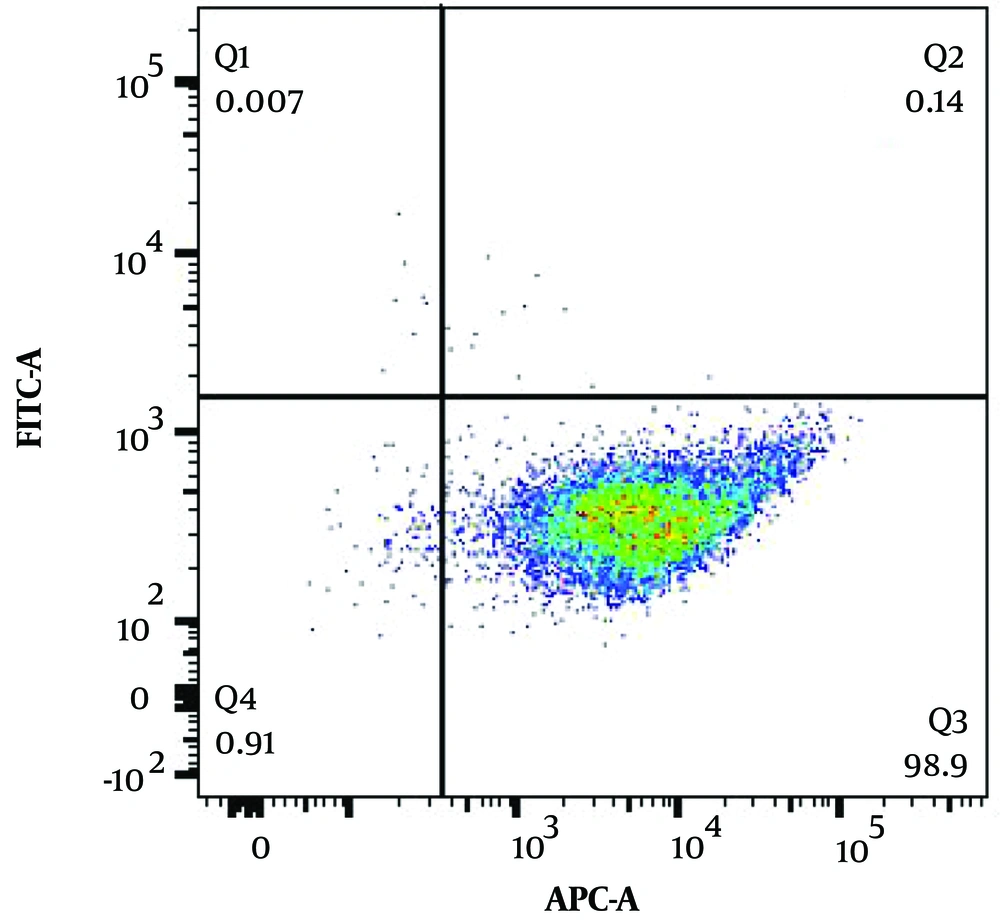
Enriched CD34+ HSCs were cultured in 15 ug/mL Notch ligand (Sino Biological)-coated culture plates containing the above-detailed cytokines that support differentiation and proliferation of HSC toward the NK cell lineage. The differentiated cells were also co-cultured with EBV-LCL cells for maximum expansion. At day 21 of the differentiation period, most of the obtained cells had an NK cell phenotype, which was demonstrated by flow cytometry as CD3-CD56+ (Figure 2).
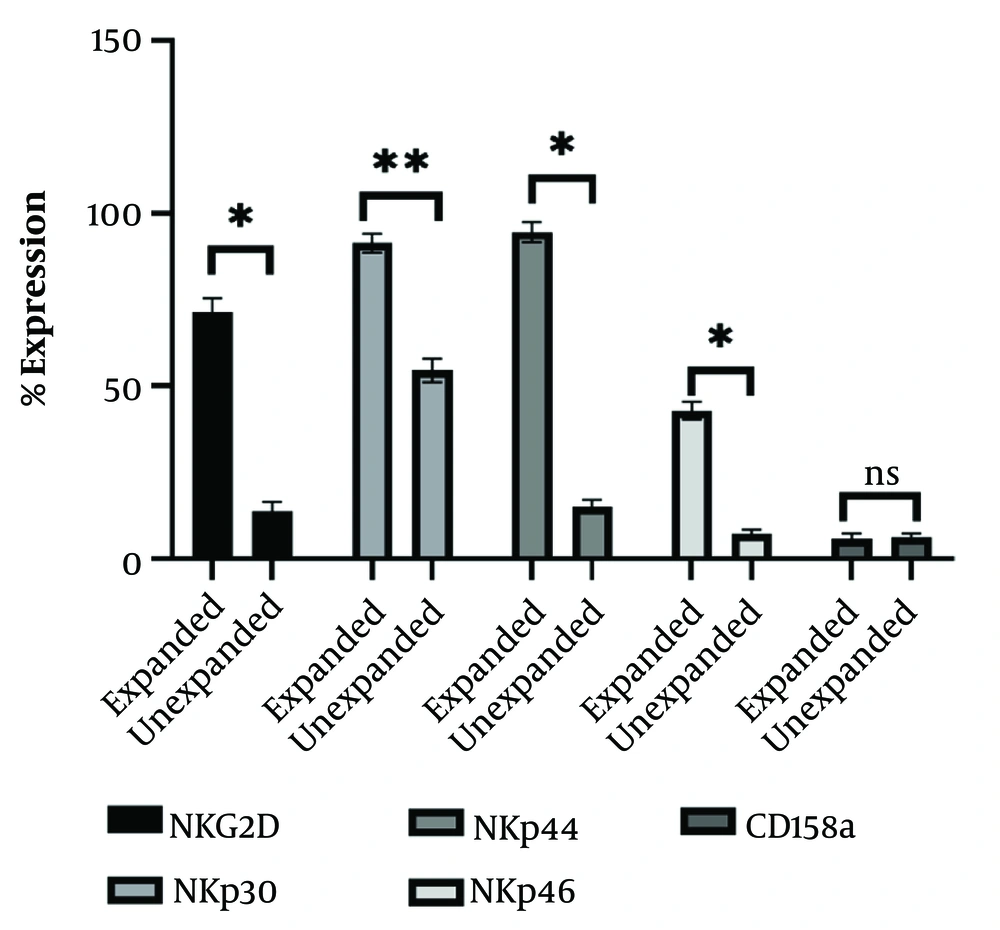
The differentiated NK cells-derived from CB-HSCs at day 21 were also tested for the expression of key NK cells activating receptors. As presented in Figure 3, there are significant differences between expanded (incubated for activation) and unexpanded (early differentiated) NK cells in terms of NKG2D, NKp30, NKp44 and NKp46, however, no significant changes were observed in the expression of the inhibitory CD158a receptor.
The expression of activating and inhibitory receptors on the natural killer (NK) cells derived from cord blood hematopoietic stem cells (CB-HSCs). Expanded (activated cells: Day 28) and unexpanded (early differentiated: Day 21) NK cells were examined by flow cytometry and the mean ± SD of at least of triplicate experiments are presented. *: P < 0.05; **: P < 0.01
4.3. CB-HSC-Derived NK Cells Exert Anti-tumor Activities
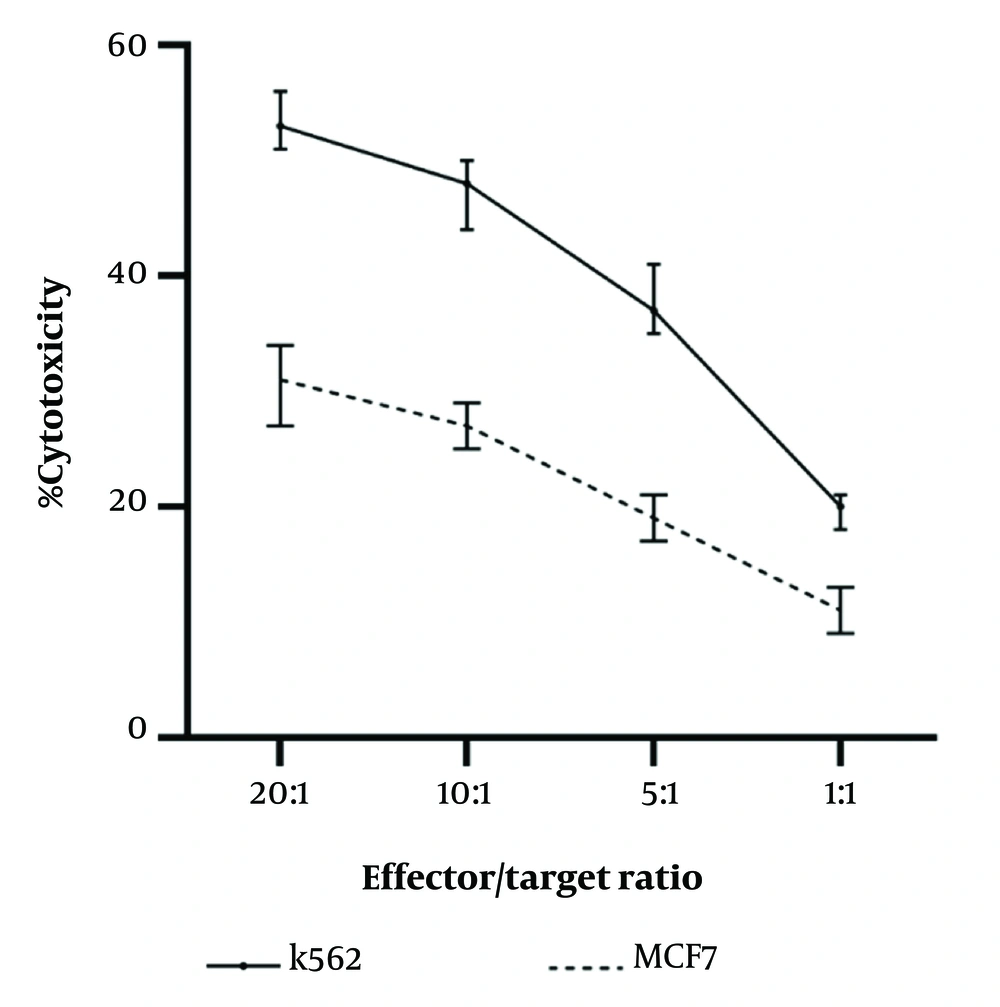
We have also performed functional assays to test the potential anti-tumor activity of the CB-HSCs-derived NK cells. To this end, the obtained NK cells (effector cells) cocultured with the breast cancer MCF7 cells and K562 cells (target cells) at different effector: Target ratios. Based on the obtained results, CB-HSC-derived NK cells were capable of inducing cytotoxic effects on both K562 and MCF7 cells (Figure 4). We observed that with the increase of effector to target ratio (E: T) to 20:1 remarkable cytotoxic effect was achieved.
The cytotoxic activity of the cord blood hematopoietic stem cell (CB-HSC)-derived natural killer (NK) cells against K562 and MCF7 tumor cells. Different effector: Target ratios (1:1, 5:1, 10:1, 20:1) were employed in the experiments. The mean ± SD of the at least two triplicate experiments are presented.
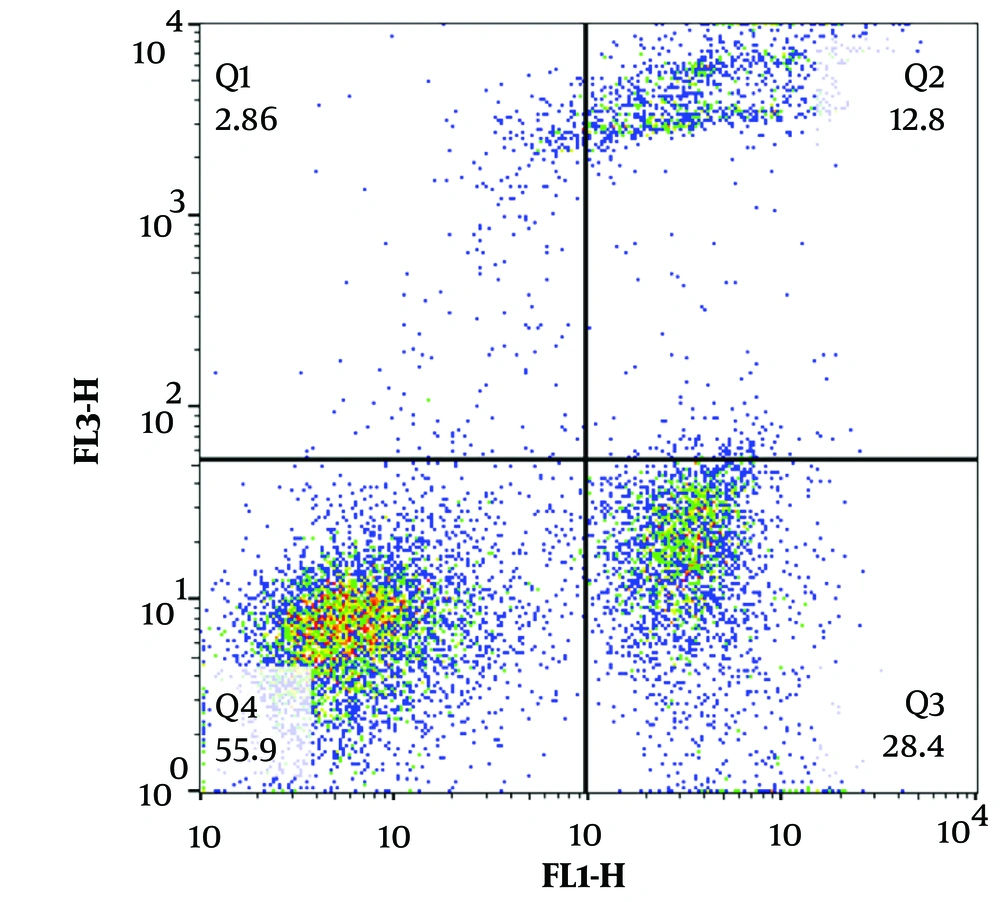
We also tested whether CB-HSC-derived NK cell-induced cytotoxicity is mediated by apoptosis. As shown in Figure 5, the Annexin V/PI assay results demonstrate that NK cells were able to kill MCF-7 cells (detected as early apoptosis 28.4%). Moreover, NK cell-induced late apoptosis rate was 12.8% in MCF-7 breast cancer cells.
Cord blood hematopoietic stem cell (CB-HSC)-derived natural killer (NK) cells induce apoptosis in MCF7 cells. Annexin V/PI testing was done and the graph was a representative of triplicate experiments. Annexin V+ cells were identified as early apoptosis, while Annexin V+/PI+ cells were defined as late apoptosis.
4.4. CB-HSC-Derived NK Cells Release IFN-γ and TNF-α
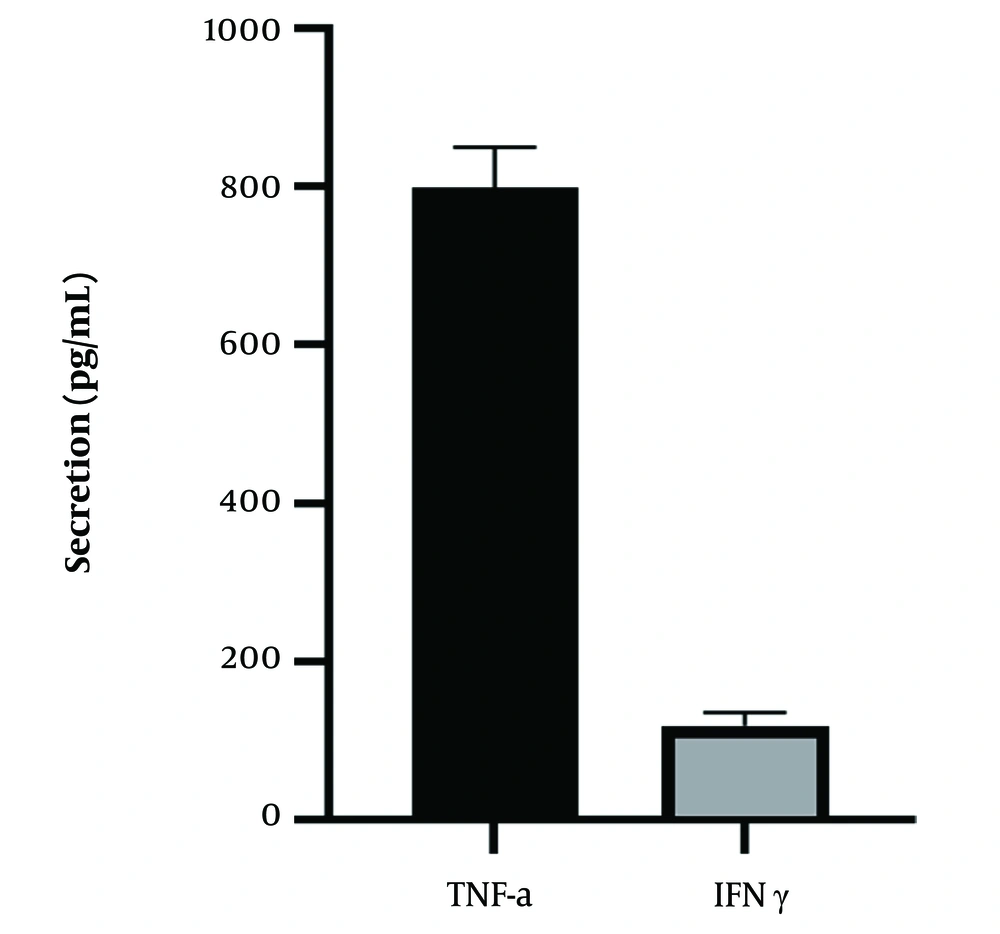
We also tested the expression of key cytokines released by NK cells. In this regard, CB-HSC-derived NK cells were experienced with tumor cells and then their supernatant was examined for the release of IFN-γ and TNF-α. As can be found in Figure 6, CB-HSC-derived NK cells could release significant amounts of IFN-γ and TNF-α in response to their exposure to the tumor cells.
5. Discussion
Although the use of NK cells in cancer immunotherapy has gained strong attention during the last decades, attaining large-scale off-the-shelf products was the main challenge. To circumvent this obstacle in natural killer cell-based immunotherapy, numerous attempts have been made to use stem cells to produce NK cells. There are several reports in the literature that demonstrate that CB-HSC could differentiate into NK cells with potential anti-tumor activity, however different methods are described (14). Therefore, in the present study we aimed to set up a protocol to differentiate natural killer cells from umbilical cord blood haematopoietic stem cells and evaluate their anti-tumor activity against breast cancer human tumor cells.
In the present study, we employed a cytokine cocktail to differentiate CD34+CB-HSCs toward NK cells. We isolated CD34+ cells from CB with high purity (Figure 1) and then used them for the differentiation of NK cells. As shown in Table 1 and Figure 2, CD34+ cells were cultured for 21 days under the differentiation condition to produce NK cells (CD56+ CD3- cells). Our results showed that the cytokine cocktail used in this study is capable of differentiating NK cells. Previous studies have also reported that cord blood CD34+ cells can be differentiated from NK cells. For instance, Pinho et al. have employed a cocktail of IL-2, IL-3, IL-7, SCF and Flt3-L cytokines to differentiate NK cell from CB-CD34+ cells (15). They found that this cytokine cocktail can induce NK cells differentiation, however, the culture condition does not affect the expression of KIRs and NCRs (15). Others have also shown that a cocktail of cytokines including FlT3-L, SCF, IL-7 and IL-15 in combination with IL-21 or IL-12 could improve maximum differentiation of NK cells and promote their cytokine release, cytolytic function and the expression of KIRs (16). The addition of other soluble factors (TPO, G-CSF, IL-6) has also been suggested to further support precursor proliferation from CB samples (17-19). In another study, different combinations of cytokines and feeder cells were employed to differentiate NK cells from HSCs. It was found that the presence of FlT3-L, SCF, IL-7, and IL-15 as well as feeder OP9-DL1 cells could lead to the generation of NK cells (20). Regarding the previous reports and our findings it can be inferred that a cytokine cocktail could provide a condition for NK cell differentiation without a need for feeder stroma.
Although the differentiation of NK cells from CB is of importance, whether the differentiated cells are functional or not should be considered. Here, we employed both IL-15 and IL-2 to expand the differentiated NK cells. As shown in Figure 3, the cytokine-expanded NK cells express significantly elevated levels of the activating NKG2D, NKp30, NKp44 and NKp46 receptors. Similar to our findings, others have also shown that the use of expansion protocol in HSC-derived NK cells could increase their numbers as well as functionality. For instance, Spanholtz et al. revealed that expanded CD34+CB cells could easily differentiate into CD56+CD3- NK cells at least with a mean expansion rate of > 2,000 fold and more than 90% purity (18). It was also shown that the low dose of human IL-15 drives CB-NK cell expansion in vivo (21). For example, CB-NK cells treated with IL-15 were shown to potently inhibit K562 cells growth in vivo, demonstrating that IL-15 could improve the anti-tumor activity of CB-NK (21). There are reports showing that such a response can also be achieved by co-administration of NK cell and IL-2 (22, 23). Considering this, we have also included IL-2 to further improve CB-HSC-derived NK cells functionality. Previous studies have also demonstrated that the combination of IL-2 and IL-15 could robustly increase the expression of activating receptors including NKp30, NKp44 and NKp46 and NKG2D on NK cells (11). This was confirmed by our findings demonstrating that IL-15 and IL-2 treated -CB-HSCs-derived NK cells expressed higher levels of NKG2D, NKp30, NKp44 and NKp46 compared to those differentiated NK cells that were not treated for expansion.
Examining the literature, it can be conceived that IL-12 might be the best combination with IL-15, however, the results achieved are not consistent. For example, a previous study showed that the replacement of IL-2 by IL12 could enhance IFN-γ production and the anti-tumor activity of HSC-NK cells against AML cells in vitro and in vivo (24). It was also shown that IL-12 could increase the number of NKG2A- and KIRs-expressing HSC-NK cells, which are more sensitive to the stimulation of target cells (24). However, in contrast, other studies have shown that NK cells derived from CB-CD34+ and PB-CD34+ cells did not respond similarly to this cytokine, and IL-12 could not improve the cytolytic activity of the CB-CD34+-NK cells compared to those PB-CD34+NK cells (25).
We have also examined the anti-tumor effects of the NK cells derived from CB-HSCs. Two tumor cell lines, including NK-sensitive MHC-I-deficient K562 leukemia cells, and MCF7 a human breast cancer cell line, were used to examine the anti-tumor effects of the CB-HSC-derived NK cells. The results showed that the obtained NK cells could remarkably destroy tumor cells, however, the antitumor effects of the CB-HSC-derived NK cells were more obvious at higher effector-to-target rations (Figures 4 and 5). Interestingly, the CB-HSC-derived NK cells were also able to produce higher amounts of TNF-α and IFN-γ in response to exposure to K562 cells (Figure 6). Consistent with our findings, previous studies have also shown that CB-HSCs-derived NK cells release higher levels of IFN-γ and could lyse tumor cells more robustly than PB-HSCs-derived NK cells (25, 26).
5.1. Conclusions
In this study, CD34+ HSCs isolated from cord blood were tested to differentiate into NK cells. Our findings revealed that the CB-HSC-derived NK cells are capable of killing tumor cells. Interestingly, the results revealed that treatment of newly differentiated NK cells with a pair of IL-15 and IL-2 for at least 7 days could robustly increase their expression of activating NKG2D, NKp30, NKp44 and NKp46 receptors, promoting their anti-tumor activities. However, further research to set a large-scale production protocol is needed. Moreover, future research on the capability of CB-HSCs-derived NK cells to produce CAR-NK cells would also be of interest. In conclusion, our results support that CB-HSCs-derived NK cells could be considered a promising immunotherapeutic option in cancer treatment.