1. Context
Breast cancer (BC) is the most common cancer in women in the world. BC caused 685 000 deaths worldwide in 2020. About half of BC occurs in women, who have no specific risk factors other than gender and age (1).
Approximately 15 to 30% of patients with early-stage breast cancer (ESBC) have human epidermal growth factor receptor 2 (HER2) protein overexpression and/or HER2 gene amplification positive disease, which is associated with a poor prognosis (2-4).
Neoadjuvant chemotherapy is used for patients with early and advanced BC. It is also used for locally advanced BC, inflammatory BC, and removal of large tumors to allow breast-conserving treatment (5). Neoadjuvant chemotherapy can also serve as an in vivo testing of chemotherapy sensitivity (6). Furthermore, neoadjuvant chemotherapy provides an opportunity to individualize treatment and an opportunity to assess response to a regimen on a patient-by-patient basis and provide prognostic information based on clinical and pathologic response (5, 7).
In recent decades, neoadjuvant chemotherapy has gained considerable importance for the treatment of BC. There are several neoadjuvant treatment strategies for HER2-positive BC. In addition, drugs are used in the neoadjuvant setting (8). There are various agents to target HER2 positive including trastuzumab, pertuzumab, and antibody-drug combinations (such as T-DM1). These targeted drugs have significantly changed the prognosis of patients with HER2-positive BC over time (9). Since these biological agents are antibodies not chemicals, they are effective but expensive. When these treatments are targeted, they are combined with chemotherapy to be effective in killing cancer cells (1).
Several economic evaluation studies have been conducted regarding neoadjuvant treatment strategies for HER2-positive BC. Each of these studies has presented different findings and examined different aspects. Collecting various economic evaluation evidence on these strategies will help clinicians, policymakers, decision-makers, and other stakeholders to make decisions about resource allocation, prioritization, and optimization of clinical outcomes.
2. Objectives
Therefore, this study aimed at collecting and interpreting economic evaluation studies related to neoadjuvant treatment strategies for HER2-positive BC.
3. Methods
3.1. Design
This systematic review was conducted according to the guidelines for conducting reviews as outlined in the Cochrane Handbook (10). The collection and reporting of the study included the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines (11).
3.2. Study Search
An unrestricted literature search was conducted between May and October 2023 in the following electronic databases: PubMed, Web of Science (WOS), ScienceDirect, and Scopus. Google Scholar search engine was used to complete the search process. The search was restricted to English-language publications. Using MeSH (Medical Subject Headings), the following terms were searched together with Boolean operators: (“Breast Cancer” OR “Breast Neoplasm”) AND (“Neoadjuvant”) AND (“Economic Evaluation” OR “Cost-Effectiveness” OR “Cost-Utility” OR “Cost-Consequences” OR “Cost-Benefit”) AND (“HER2”).
3.3. Study Selection and Data Extraction
Two authors (MMJ, MSN) independently selected studies based on inclusion and exclusion criteria and extracted detailed data from eligible studies. Any disagreement regarding eligibility was resolved by consensus.
The inclusion criteria were as follows:
• Studies that compare the cost of at least two strategies
• Studies published in English
The exclusion criteria were as follows:
• Studies, where the outcome was not clearly stated
• Studies, whose data could not be extracted
• Studies that included duplicate data
The items for data extraction were the year of publication, first authors, type of study, study setting, characteristics of participants, model, perspective, time horizon, sample size, strategies/ regimens, outcome/outcomes, and findings. Any dispute regarding study selection and data extraction was resolved by consensus and, if necessary, refereed by a third author.
3.4. Quality Assessment
To assess the quality of the studies, the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines (12) were used, which evaluate 24 criteria. The included studies were assessed for quality and classified into 3 categories including high (75% or more), moderate (from 50 to 74.9%), and low quality (less than 50%) (13, 14).
3.5. Data Synthesis
To provide an understandable summary of the included evidence, study characteristics were reviewed and key findings were categorized and presented. The categories of key elements in each study were discussed and agreed upon by the authors. The results of the included studies were tabulated and descriptively summarized. As this review is descriptive, numerical data were not extracted for statistical analysis.
4. Results
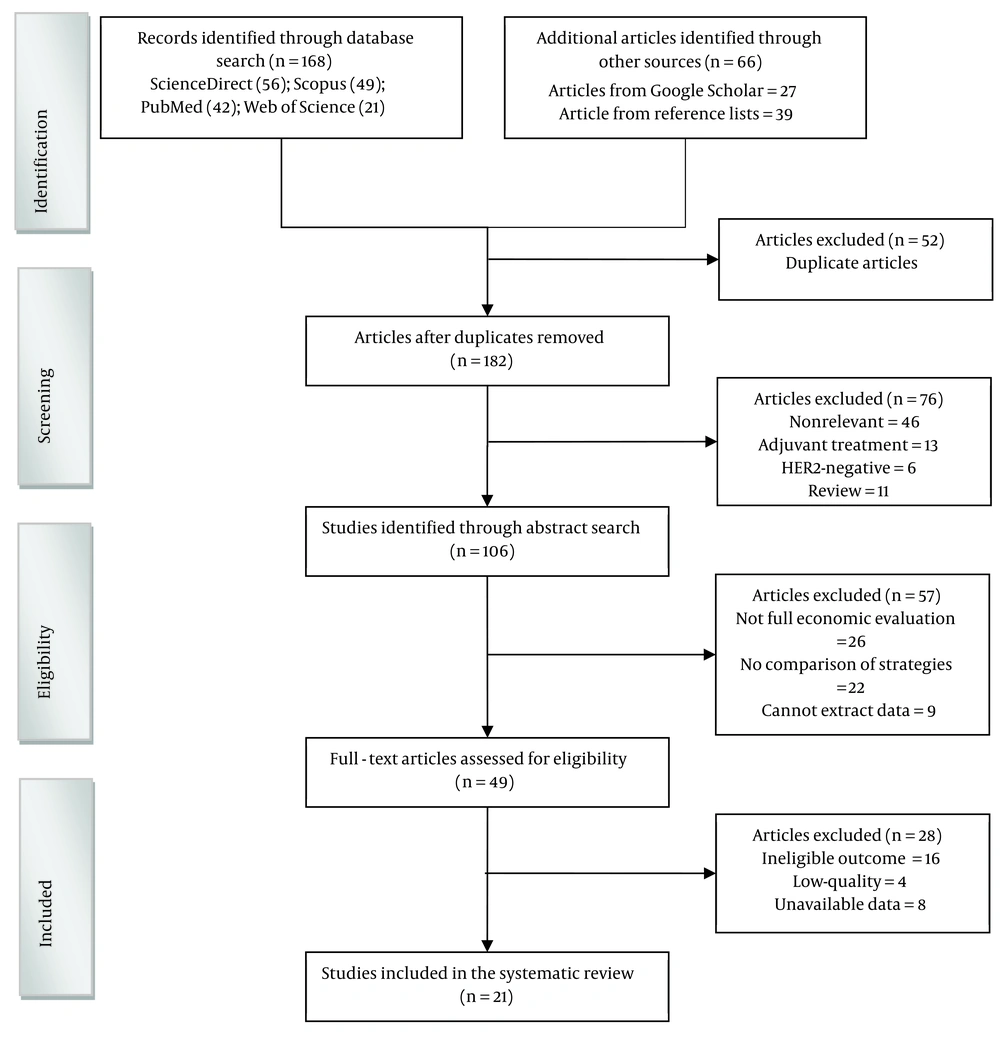
From 234 unique citations identified through a literature search, 49 full‐text records were reviewed. Of these, 21 studies have been included and reviewed. The flow of studies through the screening process is presented in Figure 1.
4.1. Characteristics of Included Studies
Table 1 summarizes the characteristics of the included studies. The publication year of the included studies was from 2014 to 2023. Twenty-one studies were included from 14 countries. Seven studies were conducted in the United States (3, 15-20), 2 studies were conducted in Taiwan (21, 22), and 12 other studies were conducted in Brazil (23), Canada (24), China (25), Germany (26), Italy (27), Japan (28), Macedonia (29), Mexico (30), Portugal (31), Russia (32), Singapore (33), and Spain (34).
| Author/Authors (References) | Year | Setting | Type of Study | Patients | Model | Perspective | Time Horizon | Strategies/Regimens | Outcome/Outcomes | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Hassett et al. (15) | 2020 | United States | Economic Evaluation | Stage II–III HER2-positive BC | Decision-analytic | Payer (Medicare) | 5 years | Two de-escalated regimens; (1) T-DM1; (2) TH; Three intensive regimens: (1) TCHP; (2) THP+ AC; (3) THP | QALYs and costs | Among the de-escalated strategies: TH was the most cost-effective; Among intensive neoadjuvant strategies, THP treatment was more effective and less costly than TCHP or THP + AC.; For HR-positive cancer, neoadjuvant TH dominated the THP strategy. |
| Hendrix et al. (3) | 2022 | United States | Cohort & Economic Evaluation | High-risk HER2-positive ESBC | Decision-analytic | - | 10 years | (1) A group of patients treated with T-DM1; (2) A hypothetical group without disease recurrence | Recurrence, BC deaths, direct medical costs, and costs due to lost productivity | Despite the impressive benefits of T-DM1 therapy in patients with HER2-positive ESBC, those who do not achieve PCR face a clinically significant risk in the next 10 years, especially in the first 5 years after treatment. |
| Kunst et al. (16) | 2020 | United States | Economic Evaluation | ERBB2-positive BC | Decision-analytic | Payer | - | (1) HP; (2) THP; (3) DDAC-THP; (4) TCHP | Lifetime costs, QALYs and; ICER | Strategy 3 was associated with the lowest costs ($415,833) and the highest health benefits (10.73 QALYs), dominating all other strategies. |
| Sussell et al. (17) | 2022 | United States | Economic Evaluation | High-Risk HER2-Positive ESBC | Hybrid decision-tree/Markov | - | - | (1) Adjuvant targeted therapy (T-DM1 or H) in the case of RD; (2) Neoadjuvant targeted therapy (infused PH versus subcutaneous FDC of pertuzumab and trastuzumab versus trastuzumab alone (H)); (3) Use of branded or biosimilar H; (4) Adjuvant targeted therapy if PCR is achieved (PH, FDC, or H) | Costs, non-metastatic recurrence, remission, invasive disease-free, and death. | Dual targeted therapy strategy via FDC (T-DM1 in the case of RD) is a cost-effective treatment. |
| Attard et al. (24) | 2015 | Canada | Cost-utility analysis | HER2-positive early BC | Markov | Payer | 28 years | (1) With Pertuzumab; (2) Without Pertuzumab | Life-years, QALYs, and direct medical costs | Given the reasonable cost and improved clinical effectiveness per QALY, the addition of pertuzumab in the neoadjuvant setting is a desirable treatment option for HER2-positive ebc patients. |
| Krawczyk et al. (26) | 2023 | Germany | Compare the treatment costs | HER2-positive early BC | - | Oncological outpatient clinic of a certified breast center at a university hospital | - | (1) Trastuzumab / pertuzumab; (2) Ttrastuzumab; (3) T-DM1 | Cost | The T-DM1 treatment strategy is associated with a 30% lower contribution margin than the other two strategies. |
| Borges et al. (31) | 2021 | Portugal | Cost estimate | HER2-positive BC | Binary logistic regression | Hospital | - | AC-DH regimen comprised 8 cycles of neoadjuvant therapy (4 cycles of cyclophosphamide + doxorubicin followed by 4 cycles trastuzumab + ofdocetaxel) (2012 – 2015); 2-AC-DHP regimen included also pertuzumab as neoadjuvant treatment (2015 - 2017) | ICER | The findings obtained in the AC-DHP group, which included Pertuzumab as a neoadjuvant treatment, showed better clinical outcomes compared to the AC-DH group |
| Zambelli et al. (27) | 2023 | Italy | Cost-consequence analysis | High-risk HER2-positive early BC | Markov | Societal | 5 years | (1) PTC; (2) TC | Life of years, QALY, direct costs, indirect costs, cumulative incidence of loco-regional/ distant recurrences | PTC strategy can be a cost-saving option for patients with a high risk of recurrence. |
| Kashiura et al. (23) | 2019 | Brazil | Estimate the economic impact | HER2-positive BC | - | - | 5 years | (1) PTC; (2) TC | Direct medical costs | PTC strategy in neoadjuvant therapy shows cost savings in patients' next-line treatments. |
| Kapedanovska Nestorovska et al. (29) | 2018 | Macedonia | Cost-utility | HER2 positive locally advanced, inflammatory, or early-stage BC | Markov | - | 20 Years | (1) PTD; (2) TD | QALYs, direct costs, and ICER | PTD is a potentially cost-effective treatment option for HER2-positive BC. |
| Colomer et al. (34) | 2016 | Spain | Cost-utility | HER2-positive BC | Markov | - | - | (1) PTD; (2) TD | QALYs | Combination of PTD in patients receiving neoadjuvant therapy results in QALY gain and cost savings. |
| Wang et al. (25) | 2021 | China | Cost-effectiveness | HER2-positive BC | Markov | payers | - | (1) PTD; (2) TD | QALYs and ICER | The PTD regimen increased patients' life expectancy and improved their quality of life, but medical costs also increased. Based on the current payment threshold in China, the PTD regime had no economic advantage over the TD regime. |
| Cheng et al. (33) | 2021 | Singapore | Cost-effectiveness | HER2-positive metastatic BC | A partitioned survival | - | - | Trastuzumab biosimilar and docetaxel with or without pertuzumab | QALYs and; ICER | Trastuzumab biosimilar and docetaxel with pertuzumab reduced the cost, but the ICER was high and not cost-effective in the Singaporean context. |
| Moriwaki et al. (28) | 2021 | Japan | Economic Evaluation | HER2-positive metastatic BC | A partitioned survival | Japanese healthcare system | 20 years | (1) PTD; (2) TD | QALYs, ICER, overall survival, regression-free survival, and direct medical costs | Treatment with PTD will not be as cost-effective as first-line therapy. |
| Durkee et al. (19) | 2016 | United States | Cost-effectiveness | HER2 overexpressing metastatic BC | Markov | - | - | docetaxel plus trastuzumab (TH) with or without pertuzumab | QALYs, ICER and medical costs | THP was not cost-effective in patients with metastatic HER2-positive breast cancer in the US context |
| Diaby et al. (18) | 2016 | United States | Cost-effectiveness | HER2-positive metastatic; BC | Markov | - | (1) THP as first-line therapy; (2) T-DM1 as second-line therapy; (3) lapatinib/capecitabine third-line therapy | Progression-free survival, costs, QALYs, ICER | THP as first-line therapy and T-DM1 as second-line therapy requires at least a 50% reduction in the total drug cost to be considered a cost-effective strategy. | |
| Leung et al. (22) | 2018 | Taiwan | Cost-effectiveness | HER-2 positive metastatic BC | Markov | National Health Insurance | (1) PTD; (2) TD | QALYs, costs in New Taiwan dollars (NT$), and ICER | First-line treatment of PTD will be cost-effective, but only assuming optimal drug cost. | |
| Diaby et al. (30) | 2017 | Mexico | Economic evaluation | HER2-positive metastatic; BC | Markov | Public and private payer | Weekly over their remaining life expectancies within the model | Four different HER2-targeted treatment sequences | Progression-free survival, costs, QALYs, and ICER | Using three or more lines of trastuzumab in combination with other regimens, but not with T-DM1 or pertuzumab, is the most cost-effective option. |
| Babigumira et al. (20) | 2014 | United States | Cost-effectiveness | HER2+, locally advanced, inflammatory, or early BC | Combined decision-analytic and partitioned survival | - | - | (1) TH; (2) THP; (3) HP; (4) TP | QALYs, cost, drug monitoring, drug administration, clinical management of adverse events, and progressive disease (PD) | THP was predicted to be cost-effective in the neoadjuvant setting. |
| Ignatyeva and Khachatryan (32) | 2016 | Russia | Cost-utility | Locally Advanced, Inflammatory, or Early HER-2-positive BC | Markov | Payer | 4 weeks | (1) THP; (2) TH | QALY and ICER | Neoadjuvant treatment with THP is an efficient option for treating patients. |
| Diaby et al. (21) | 2020 | Taiwan | Cost-effectiveness | HER-2 positive metastatic BC | Markov | Taiwanese National Health Insurance Administration’s (TNHIA) | Over a lifetime | Four treatment sequences | Disease progression, transition probabilities, probability of adverse events, Costs and QALYs | The first-line as trastuzumab plus docetaxel, and then in the second and third-lines, the use of TDM1 and trastuzumab plus lapatinib are the most cost-effective strategies. |
The economic evaluation analysis model used in the studies was different; 11 studies have used the Markov model (18, 19, 21, 22, 24, 25, 27, 29, 30, 32, 34), 3 studies have used the decision-analytic model (decision trees) (3, 15, 16), 2 studies have used partitioned survival (28, 33), 1 study has used Binary logistic regression model (31), 1 study has used combined decision tree (decision-analytic) and partitioned survival (area under the curve) (20), 1 study has used hybrid decision-tree/Markov (17), and 2 studies have not used any model (23, 26).
Among the included studies, 8 studies have used the payer perspective (15, 16, 21, 22, 24, 25, 30, 32), 1 study has used the healthcare system perspective (28), 1 study has used the hospital perspective (31), 1 study has used the societal perspective (27), 1 study has used the oncological outpatient clinic perspective of a certified breast center at a university hospital (26), and 9 studies have not considered any prospect for economic evaluation (3, 17-20, 23, 29, 33, 34). Time horizons ranged from 4 weeks (32) to a lifetime (21). Three studies used a 5-year horizon (15, 23, 27).
4.2. Quality of the Studies Included
Based on the CHEERS checklist, 16 studies (3, 15-22, 24, 25, 27, 28, 30, 32, 33) were evaluated as high quality (75.0% or more) and 5 studies (23, 26, 29, 31, 34) as moderate quality (from 50.0 to 74.9%). Among the included studies, no study was evaluated as low quality (less than 50.0%).
4.3. Description of the Strategies
In 21 included studies, 58 strategies or regimes were analyzed for economic evaluation. THP (taxol, trastuzumab, pertuzumab) in 8 studies, PTD (Pertuzumab in combination with trastuzumab and docetaxel) in 5 studies, TH (docetaxel and trastuzumab) in 5 studies, T-DM1 (trastuzumab emtansine) in 4 studies, and HP (trastuzumab plus pertuzumab) have been investigated in 3 studies.
5. Discussion
Based on the literature review, this is the first systematic review study on the cost and cost-effectiveness of nonadjuvant treatment strategies for HER2-positive BC. Twenty-one studies met the eligibility criteria and were included in this systematic review. The findings of the present study showed that the payer's perspective was used more and the most common types of models were Markov models, decision trees, and partitioned survival. Overall, 16 studies were rated as having high reporting quality (75 - 96%).
The results of this study showed that PTD is a potentially cost-effective treatment option for HER2-positive BC. Wang et al.'s (25) study found that not only the PTD regimen increased patients' life expectancy and improved their quality of life, but also increased medical costs. Therefore, it has no economic advantage over the TD method. Kapedanovska Nestorovska et al. (29) showed that PTD is a potentially cost-effective treatment option. Colomer et al.’s research (34) showed that a combination of PTD leads to increased QALYs and cost savings. Leung et al.'s study (22) had a different finding and showed that PTD would be cost-effective as a first-line treatment for HER-2 positive mBC, but only under favorable drug cost assumptions. Moriwaki et al.'s study (28) had a contrasting finding, suggesting that treatment with PTD would not be as cost-effective as first-line therapy.
The results of this study showed that one of the dominant strategies is THP. Hassett et al.’s study (15) showed that treatment with THP (among intensive neoadjuvant strategies) was more effective and less expensive than TCHP or THP + AC. Kunst et al. (16) showed that the DDAC-THP strategy was associated with the highest Health Utilities (10.73 QALYs) and the lowest costs ($415,833), dominating all other strategies. Diaby et al.’s study (18) showed that the treatment strategy with THP as the first-line followed by the T-DM1 treatment strategy as the second-line requires at least a 50% reduction in the total cost of drug preparation to be considered a cost-effective strategy. Ignatyeva and Khachatryan's study (32) showed that neoadjuvant treatment with THP is an efficient treatment option. Babigumira’s study (20) found that the THP regimen, in addition to being clinically effective, would be economically desirable in the United States. The results of Durkee et al.’s study (19) had a different finding, showing that THP was not cost-effective in patients with metastatic HER2-positive BC in the United States.
The findings of this study showed that one of the effective strategies in the treatment of patients with HER2-positive ESBC is T-DM1. Hendrix et al.’s study (3) showed that despite the significant benefits of T-DM1 therapy, those who do not achieve PCR, face significant clinical risk over the next 10 years, particularly in the first 5 years after treatment. Diaby et al.’s study (21) showed that the first line as trastuzumab plus docetaxel, and then in the second and third lines, the use of TDM1 and trastuzumab plus lapatinib are the most cost-effective strategies. Sussell et al.’s study (17) showed that dual-targeted therapy via FDC (with transfer to T-DM1 in case of RD) is a cost-effective treatment strategy. Krawczyk et al. (26) showed that the T-DM1 treatment strategy is associated with a 30% lower contribution margin than the other strategies (Trastuzumab with or without pertuzumab).
Studies have shown that neoadjuvant treatment did not differ much in terms of overall survival and disease progression compared to adjuvant treatment (35). Another study showed that HER2+ exosomes benefit tumor progression by suppressing trastuzumab-induced tumor growth inhibition and Natural Killer cell cytotoxicity. Also, simultaneous blocking of exosome release is an effective approach to improve the therapeutic effects of trastuzumab, and potentially other HER2-directed mAbs (36). Other studies have shown that BC cases with ER-/HER2+ tumors had shorter survival than ER+/PR+/HER2- tumors (37).
5.1. Limitations
Limitations of this study include significant heterogeneity in the design and treatment strategies/regimens used in each study.
5.2. Conclusions
The results of this systematic review study show that the reported strategies in the treatment of HER2-positive BC patients are cost-effective in different settings. While the findings of this review provide largely positive results, the characteristics of each strategy should be considered.
Although the overall quality of the included studies was good, future economic evaluations should further improve their methods, especially as the perspectives of other stakeholders should be considered.