1. Background
Cancer is the second leading cause of death throughout the world (1, 2) and its burden has doubled within the last 30 years (2). Breast cancer is the most common type of cancer accounting for 23% of all cancers (3), which ranks third in the most lethal malignancies among women (2, 4). Generally, 16% of cancer deaths are due to breast cancer (1); therefore, breast cancer is known as a major health problem in the world. In Iran, breast cancer is the most prevalent cancer among women (5, 6), accounting for 24.4% of all malignancies (7) and its crude incidence has been 17.81 (6).
Locally advanced breast cancer (LABC) is a subset of breast cancer characterized by the most advanced breast tumors in the absence of distant metastasis. To be more precise, recent guidelines of U.S. National Comprehensive Cancer Network have described LABC as fulfilling any of the following criteria without the presence of distant metastasis (8): tumors more than 5 cm in size with regional lymphadenopathy (N1-3), tumors of any size with direct extension to the chest wall or skin, or both (including ulcer or satellite nodules) regardless of regional lymphadenopathy, the presence of regional lymphadenopathy (clinically fixed or matted axillary lymph nodes, or any of infraclavicular, supraclavicular, or internal mammary lymphadenopathy) regardless of tumor stage.
Today, LABC is managed with a combination of surgery, radiation therapy, and chemotherapy. The treatment sequence usually starts with neoadjuvant chemotherapy to shrink the tumors in the breast and lymph nodes, thereby facilitating performing surgery with better outcomes. The surgery type in LABC is usually modified radical mastectomy (MRM), but an increasing rate of patients request breast-conserving therapy (BCT) due to various reasons, mainly cosmetic issues, which of course plays an important role in the quality of life maintenance in patients (9).
2. Objectives
In this study, we aimed at comparing the outcomes of MRM and BCT regarding local recurrence rate and disease-free survival in patients with LABC after neoadjuvant chemotherapy.
3. Methods
In the present study, patients with LABC previously undergoing neoadjuvant chemotherapy referred to the cancer research center of Shohada-E-Tajrish Hospital (Tehran, Iran) between 2013 and 2015 were included. All patients referred to our clinic were included to evaluate the inclusion and exclusion criteria. Patients with: (1) tumor size more than 5 cm, (2) 4 or more involved lymph nodes (N2, N3) and (3) stage III were included in the study and categorized into the BCT and MRM groups. Family history, age, tumor markers, oncogenes, tumor suppressor genes, and 1- to 5-year survival were recorded. The exclusion criteria included the presence of distant metastasis, the multifocal lesion in one breast, extensive microcalcification in the breast, and huge tumor size in comparison to breast size, which makes breast-conserving surgery non-feasible, the presence of chemoradiotherapy and hormone therapy contraindications, and considerable skin changes.
Medical records were reviewed and questionnaires were filled out. Phone contact, clinic visits, and paraclinical tests were also conducted. Questionnaires included family history, age, tumor markers, oncogenes, tumor suppressor genes, and 1- to 5-year survival.
Data confidentiality was respected through the study. No individual report was published. Patients’ treatment did not alter due to sole research purposes. No additional charge was applied to patients. Prior to enrollment, written informed consent was obtained from all subjects and Shahid Beheshti University of Medical Sciences board of ethics approved the protocol of the study. All procedures regarding human subjects were in accordance with Helsinki Declaration guidelines.
3.1. Statistical Analysis
Statistical analyses were performed, using SPSS version 19.0 for Windows (IBM Corporation, New York, United States). Continuous variables are presented as mean ± standard error of mean and categorical variables as proportions. Baseline variables between BCT and MRM groups of the trial were compared, using independent t test or chi-square. The comparison of disease-free survival across the two treatment groups was based on Kaplan-Meyer estimates, and P values were calculated from non-parametric log-rank tests. To account for potential confounders, analyses of crude log-rank tests were corroborated with Cox regression models accounting for the following predefined potentially confounding variables. In all tests, a P value of less than 0.05 was considered necessary to discard the null hypothesis.
4. Results
In general, 115 patients were evaluated in the present study, including 56 patients (48.7%) in the BCT group and 59 patients (51.3%) in the MRM group. The mean value of age was 48.23 ± 1.51 and 48.76 ± 1.30 years in BCT and MRM groups, respectively (P = 0.79). Patients’ characteristics before surgery are shown in Table 1.
| Parameter | BCT | MRM | P Value |
|---|---|---|---|
| Breastfeeding duration, mo | 24.83 ± 4.41 | 26.33 ± 4.59 | 0.85 |
| Follow-up duration, mo | 24.48 ± 1.13 | 28.42 ± 1.03 | 0.11 |
| Pregnancy times, No. (%) | 0.683 | ||
| 0 | 25 (44.64) | 19 (32.20) | |
| 1 | 2 (3.57) | 8 (13.55) | |
| 2 | 13 (23.21) | 8 (13.55) | |
| ≥ 3 | 16 (28.57) | 24 (40.67) | |
| Marital status, No. (%) | 0.941 | ||
| Single | 13 (23.21) | 13 (22.03) | |
| Married | 40 (71.42) | 42 (71.18) | |
| Divorced | 3 (5.35) | 4 (6.77) | |
| Smoking, No. (%) | 8 (14.28) | 4 (6.77) | 0.155 |
| Abortion, No. (%) | 0.171 | ||
| 1 | 3 (5.35) | 7 (11.86) | |
| 2 | 0 (0) | 3 (5.08) | |
| 3 | 2 (3.57) | 1 (1.69) | |
| Live birth, No. (%) | 0.423 | ||
| 0 | 26 (46.42) | 19 (32.20) | |
| 1 | 3 (5.35) | 11 (18.64) | |
| 2 | 13 (23.21) | 8 (13.55) | |
| ≥ 3 | 14 (25) | 21 (35.59) | |
| Family history of breast cancer, No. (%) | 0.951 | ||
| Absent | 43 (76.78) | 44 (74.57) | |
| 1st degree relatives | 5 (8.92) | 6 (10.16) | |
| 2nd degree relatives | 8 (14.28) | 9 (15.25) |
Baseline Characteristics of Study Participants in BCT and MRM Groups
The history of hormone consumption showed a significant difference between the two groups (P = 0.032); there was a positive history of hormone consumption in 18 patients (32.14%) in the BCT group and 30 patients (50.84%) in the MRM group. The cancer stage was not significantly different between BCT and MRM groups (P = 0.551). Twenty-one (37.51%) patients of the BCT group and 19 patients (32.20%) of the MRM group were in stage II. Thirty-five patients (62.5%) and 40 (67.79%) patients were classified as stage III in BCT and MRM groups, respectively.
Hormone receptors’ status was not significantly different between the two groups. The details are seen in Table 2.
| Hormone Status (N) | BCT | MRM | P Value |
|---|---|---|---|
| ER (positive/negative) | 40/16 | 43/16 | 0.513 |
| PR (positive/negative) | 35/21 | 30/29 | 0.142 |
| HER2 (positive/negative) | 12/44 | 8/51 | 0.192 |
| P53 (positive/negative) | 7/49 | 12/47 | 0.190 |
Hormone Receptors’ Status in Breast Cancer Patients in BCT and MRM Groups
Recurrence was detected in 21 patients (18.26%). The average age in non-recurrence and recurrence groups was 48.6 ± 10.9 and 47.9 ± 10.9 years, respectively (P = 0.99). Follow-up duration was not significantly different between non-recurrence and recurrence groups (26.8 vs. 25 months, P = 0.940).
Hormone receptors’ status in two recurrence and non-recurrence groups are shown in Table 3.
| Hormone Status (%) | Non-Recurrence, %/% | Recurrence, %/% | P Value |
|---|---|---|---|
| ER (positive/negative) | 71.3/28.7 | 76.2/23.8 | 0.065 |
| PR (positive/negative) | 55.3/44.7 | 61.9/38.1 | 0.052 |
| HER2 (positive/negative) | 17/78 | 19/81 | 0.039 |
| P53 (positive/negative) | 16/84 | 19/81 | 0.039 |
Hormone Receptors’ Status in Recurrence and Non-Recurrence Groups
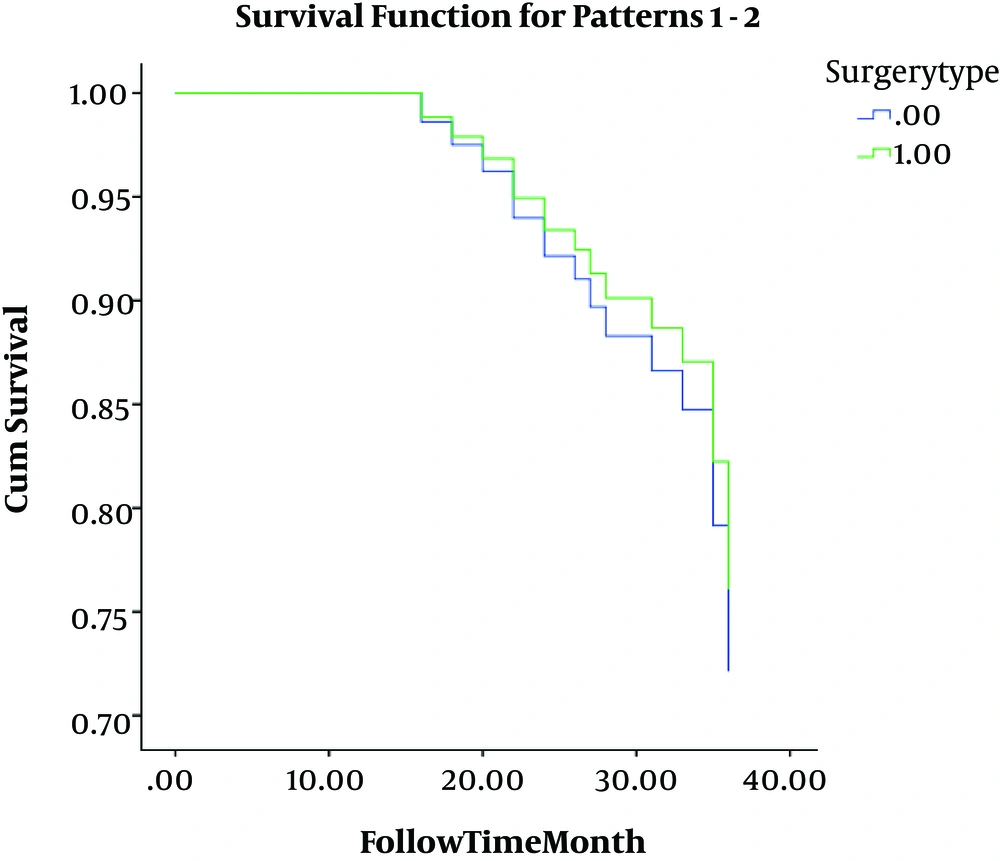
The breast cancer stage was significantly different between the two groups. Stage II comprised 37.2% of non-recurrence and 23.8% of recurrence cases (P = 0.052). Stage III was seen in 62.8% of cases of the non-recurrence group and 76.2% of recurrence group patients (P = 0.047). Hormone intake was positive in 43.6% and 33.6% of non-recurrence and recurrence group patients, respectively (P = 0.051). As shown in Figure 1, the survival rate was not significantly different between the BCT and MRM groups (P = 0.250); further analysis correlating other study parameters with survival showed that only smoking is significantly associated with survival (P = 0.041).
5. Discussion
Neoadjuvant chemotherapy can lead to the downstaging of the primary tumor. For large tumors that were planned to undergo mastectomy surgery, neoadjuvant chemotherapy has been shown to shrink the tumor and make BCT feasible (10, 11). In the current study, the local recurrence rate was compared between patients of BCT and MRM groups after neoadjuvant chemotherapy in patients with LABC. The main challenge in treating patients with LABC with BCT is a concern about the probability of a higher recurrence rate in comparison to the MRM method. It is speculated that tumors treated with neoadjuvant chemotherapy might convert into multicentric segments, which increases the risk of recurrence with the BCT method (12). There was a 21% recurrence rate in our study, which was not significantly different between the two groups.
In the Nold et al.’s study (13), 55% of patients had chosen the MRM method and the majority of them said that fear of recurrence is the reason for their choice. In the Lam et al.’s study (14), MRM patients mentioned longer survival, lower recurrence, and no need for retreatment.
Touboul et al. (15, 16) have evaluated the recurrence rates of both methods in two consecutive years and reported a 16% to 20% recurrence rate for BCT and a 5.4% to 6% recurrence rate for MRM. This difference did not reach statistical significance. The majority of available reports reveal similar findings except for Lerouge et al.’s study (17) reporting 4% and 23% recurrence rates in MRM and BCT groups, respectively, which was significantly different. The survival rate did not differ between the two groups in that study.
The disease-free survival rate was not significantly different between the two groups in our study (P = 0.250). NSABP-B18 (18) has reported a higher local recurrence rate in BCT compared to MRM, but after adjustment for age and tumor size, this significant difference was resolved. Ishitobi et al. (19) could not reveal any significant difference in recurrence-free survival according to surgery type after neoadjuvant chemotherapy. Halverson et al. (20) also emphasized that 5-year survival was 95% to 100% either in BCT or MRM regardless of age, grade, or lymph nodes’ status. A systematic review in 2016 (21) has reported that 5-year survival was lower in the mastectomy group, but this difference was not statistically significant. Vergine et al. (22) also declared that undergoing neoadjuvant chemotherapy can make BCT as successful as MRM. Contrarily, Shenkier et al. (23) have reported that standard surgery treatment in LABC is MRM and performing BCT is not allowed. Heil et al. (24) have also opposed the recruitment of BCT as standard surgery in LABC cases.
In this study, only 2 hormone receptors were significantly different between recurrence and non-recurrence groups. HER2 and p53 receptors were significantly more prevalent in patients with recurrence; meanwhile, there was no difference between two groups considering ER and PR. Sweeting et al. (25) have reported that the HER2 receptor was present in 24% of patients with recurrence and 13.1% of patients without recurrence. This difference was significant, but in contrast, 2 other studies have reported similar expressions of the HER2 receptor in recurrence and non-recurrence groups. The P53 receptor follows the same pattern. Debled et al. (26) have reported p53 receptor expression in 17% of non-recurrence patients and 26.7% of recurrence patients with a significant difference, while Parmar’s study (27) has reported a 17% and 20.3% expression rate of p53 receptor in non-recurrence and recurrence groups, respectively.
5.1. Conclusions
The aim of the present study was to evaluate disease-free survival rates after surgery between BCT and MRM groups. Surgery type after neoadjuvant chemotherapy in patients with LABC cannot alter disease-free survival duration and recurrence rate. The role of HER2 and p53 receptors were significant in recurrence, which should be investigated in future studies. BCT in patients with LABC after neoadjuvant chemotherapy is allowed according to our findings and patients can benefit from its advantages in comparison to MRM.