1. Introduction
Lung cancer stands as the leading cause of cancer-related mortality worldwide, as per GLOBOCAN 2020 (1). Epidermal growth factor receptor (EGFR) mutations represent oncogenic drivers strongly linked to the adenocarcinoma subtype of non-small cell lung cancer (NSCLC). The efficacy of EGFR-targeted therapy has been underscored by significant milestones over the past three decades, emerging as a crucial strategy against this fatal ailment. Among the well-known EGFR mutations, including Exon 19 deletions (Del19) and Exon 21 substitution L858R, which constitute 90% of cases, others such as G719X (Exon 18), L861Q (Exon21), S768I (Exon20), and Exon 20 insertion are less prevalent (2). The G719X mutation on Exon 18 is rare, accounting for approximately 2% to 4% of EGFR mutations. This mutation involves point mutations, where the glycine at position 719 is substituted with other residues, primarily alanine (G719A), cysteine (G719C), and serine (G719S) (3, 4).
Thus far, the FDA has approved three generations of TKIs for NSCLC. The 1st generation consists of reversible EGFR TKIs (erlotinib and gefitinib). The 2nd generation includes irreversible ErbB family blockers (Afatinib and dacomitinib). Since 2015, Osimertinib, a 3rd generation, irreversible EGFR wild-type sparing TKI, has been a prominent treatment option for NSCLC patients with EGFR mutations (5). However, uncommon mutations such as G719X (Exon 18) exhibit a heterogeneous response to tyrosine kinase-targeted therapy due to molecular variations across exons 18-21. This variability in response underscores the need for personalized TKI treatment strategies to maximize benefits and minimize adverse events. Post hoc analyses of the randomized LUX-lung trials have shown that Afatinib is effective against uncommon mutations including S768I, L861Q, and G719X (6). This 2nd generation EGFR-TKI has received widespread approval in over 80 countries for NSCLC treatment. However, Afatinib's tolerability is hampered by its cumulative dose. Although the initial dose in many trials is daily Afatinib 40mg, a high rate of patients experience treatment interruptions or dose reductions due to toxicity. Common gastrointestinal and skin toxicities associated with Afatinib include diarrhea (70% - 95%), rash/dermatitis acneiform (67% - 89%), stomatitis/mucositis (29% - 72%), and paronychia (10.5% - 56.8%) (3, 7). A 280mg weekly dose of Afatinib has been developed to mitigate adverse event rates compared to the standard dose. In a small group of 8 adenocarcinoma lung cancer patients with ERBB2 exon 20 insertions (20ins) mutation, approximately two-thirds of patients showed a partial response, and one case exhibited a complete response to Afatinib. Among the 8 cases, only 2 experienced diarrhea, and rash was not reported (8).
Furthermore, Afatinib has shown promise in combination with surgery. The phase II ASCENT trial demonstrated that neo-adjuvant Afatinib achieved a high objective response rate (ORR) and major surgical path response without hindering the receipt of standard-of-care chemoradiotherapy ± surgery (9). In personalized practice, Francesca Mazzoni et al. reported two cases of stage III EGFR-mutated NSCLC patients who received Afatinib for up to 3 months preoperatively and up to 4 months postoperatively. Both patients experienced local and distant relapses after 9 and 10 months (10). In this report, we present a promising outcome of an advanced-stage NSCLC patient with the rare EGFR- G719C (exon 18) mutation, who underwent lobectomy after 9 months, utilizing a 280 mg weekly Afatinib strategy.
2. Case Presentation
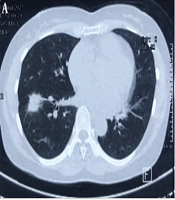
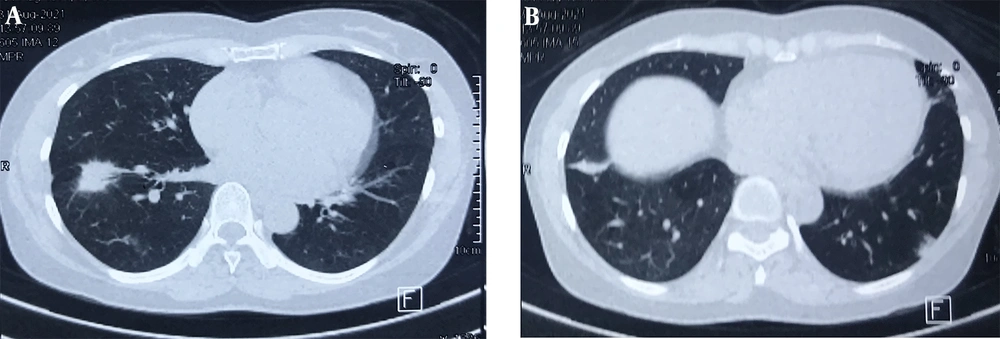
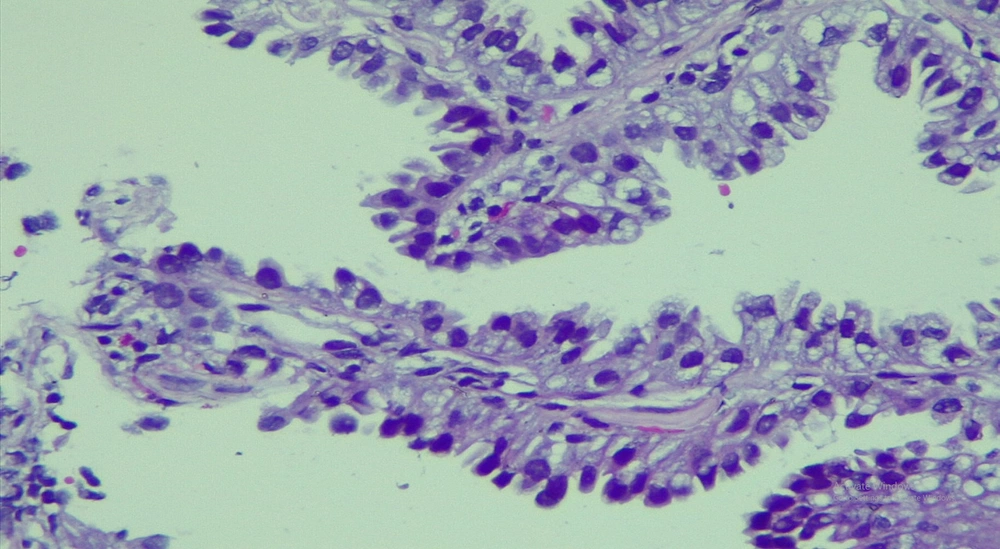
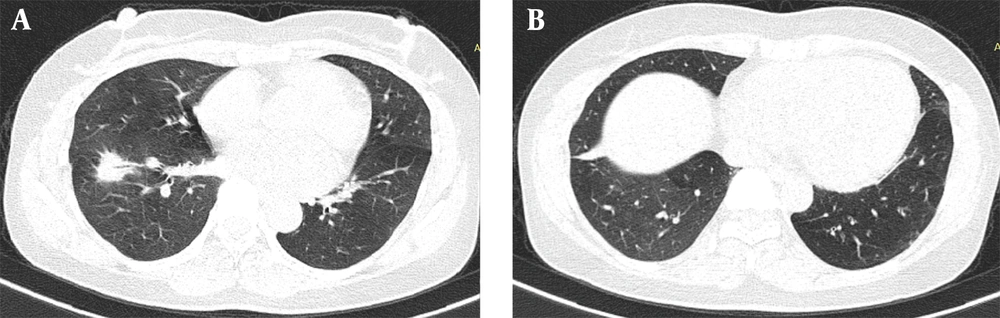
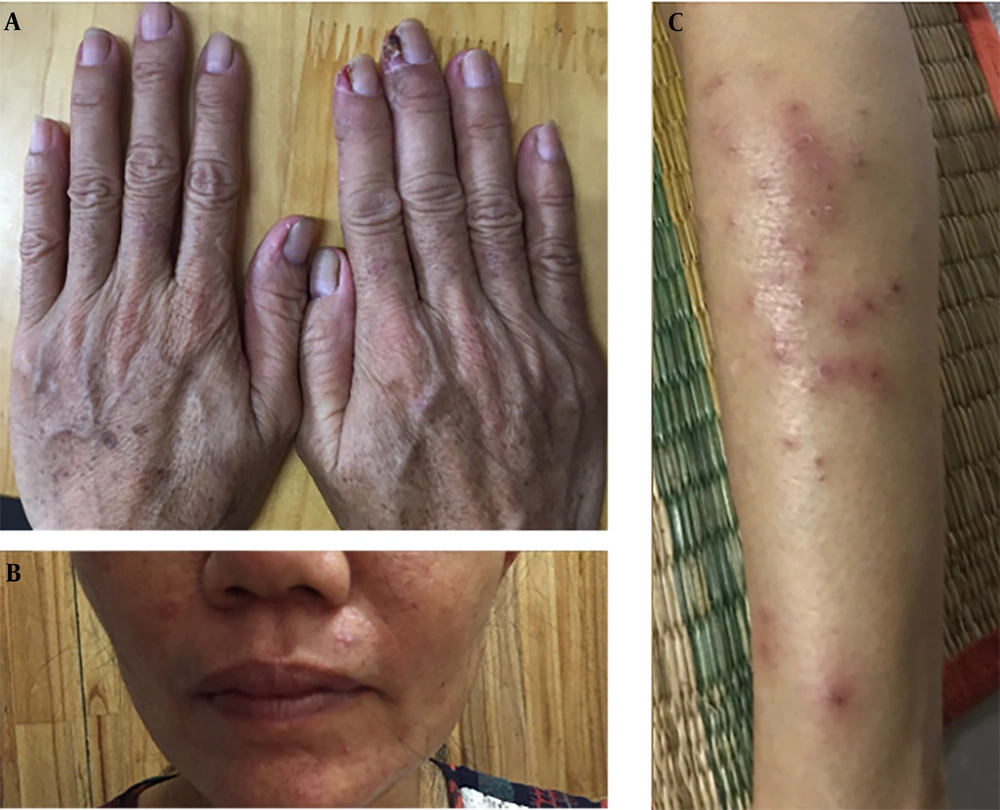
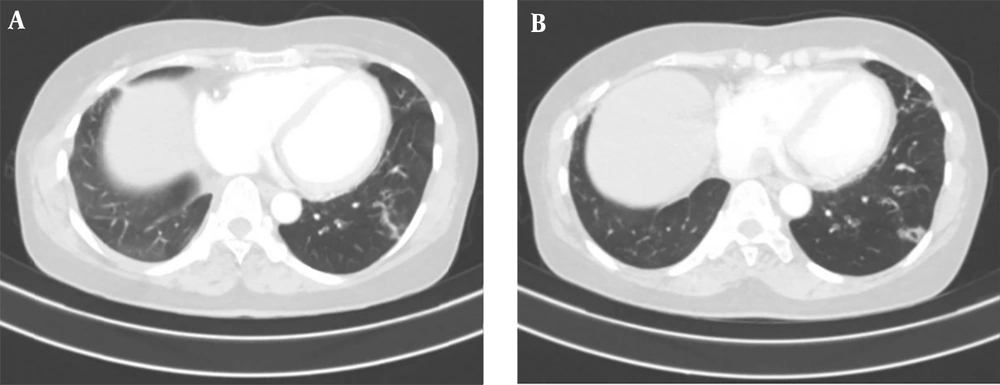
A 50-year-old non-smoking female presented with a prolonged cough and no major comorbidities. Figure 1 depicts the timeline for key events of diagnosis, treatment, and follow-up. In May 2021, she was diagnosed with a spiculated, 35mm x 35mm mass in the S6-S8 segments of the right lung, along with multiple surrounding nodules on the Computerized Tomography Scanner (CT-Scan) (Figure 2). Additionally, multiple peripheral interstitial lesions were observed in the left lung, with no reported lymph nodes. A Transthoracic Needle Biopsy (TNB) confirmed lung adenocarcinoma for the right lung tumor (Figure 3). The diagnosis indicated stage IVa right lung adenocarcinoma metastasizing to the left lung (cT3N0M1a) with the detection of the G719C mutation on Exon18 of EGFR through gene sequencing. No mutations were found in KRAS, NRAS, ROS1, BRAF, ALK, or PIK3CA. From June 2021, the patient received 280mg weekly Afatinib and underwent re-examination every 3 months with CT-Scans. By February 2022, stable disease (SD) was achieved, with nonsignificant enlargement of the lesions according to RECIST 1.1 (Figure 4). Among the adverse events (AEs) associated with Afatinib, the patient experienced mild to moderate symptoms classified using Common Terminology Criteria for Adverse Events version 5.0 (CTCAE 5.0). Diarrhea occurred on day 2 or 3 after initiating Afatinib. Dermatitis acneiform grade 2 appeared on the face and lower leg, resolving after 2 months with oral antibiotics and topical steroids. Paronychia of the hands and feet and oral mucositis were reported with grades 1 to 2 (Figure 5). In February 2022, due to a decrease in the right lung tumor size to 20x19mm and the disappearance of peripheral interstitial lesions in the left lung on CT-Scan, an open lobectomy of the right lung’s lower lobe was performed in March 2022 without complications. Histopathology and gene sequencing results matched preoperative findings, confirming lung adenocarcinoma with the G719C mutation (Exon18). Consequently, the patient continued with 280 mg weekly Afatinib post-operatively for the next 2 months but chose to discontinue treatment thereafter due to toxicities and financial concerns. Re-examinations in August 2022 and most recently in July 2023 revealed no signs of relapse in the right lung and no typical metastatic lesions in the left lung, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 after 16 months of follow-up (Figure 6).
3. Discussion
We reported a case of right lung adenocarcinoma with an EGFR mutation at the metastatic stage (left lung), initially not considered for surgery. Our diagnostic limitations included the absence of histopathological clarification for the left lung interstitial lesions. Although no lymph nodes were identified on the CT scan, confirmation through a mediastinal biopsy was warranted. However, our assessment was influenced by the risk of complications, the patient's preferences, and the availability of local facility equipment.
Given the patient's EGFR G719C mutation, TKIs were recommended as first-line therapy, despite its rarity, in accordance with practical guidelines for NSCLC in Vietnam (11). Among the Asian population, Exon18 G719x mutations have a prevalence of only 2% to 3%, with a response rate to TKIs ranging from 5 % to 66.7 % (12).
A PD-1/PD-L1 expression test was not conducted due to cost considerations associated with immune checkpoint inhibitors (ICIs) therapy, which are not covered by health insurance in Vietnam and exceed the patient's income. Additionally, previous trials involving PD1/PDL1 pathway ICIs showed that the EGFR-mutant population represented only 5 % to 14 %, with the benefits of ICIs remaining controversial in this subgroup (13).
The response to the 2nd generation EGFR TKI, Afatinib, lacks evidence to predict disease progression time. However, the safety and efficacy of Afatinib have been supported by the LUX-LUNG (LL) trial series. According to LL3 and LL6, Afatinib outperformed platinum-doublet chemotherapy in improving progression-free survival (PFS) and overall survival (OS), with a proportion of treatment-related adverse events (AEs) exceeding 15 % (7).
Several hypotheses have been proposed to explain the mechanism of response to Afatinib in patients with rare EGFR mutations.
A finding by Tsai and Tien indicated that Afatinib can lead to mitochondrial dysfunction in NSCLC cells, particularly affecting the Ca2+ influx pathway and leading to mitochondrial Ca2+ overload (14). Recent evidence supporting the role of mitochondrial metabolism in cancer survival could potentially justify the favorable response to Afatinib in our patient (15). Additionally, the effect of Afatinib on mitochondria may enhance the response to immune checkpoint inhibitors by upregulating PD-L1 expression in cancer cells (16).
In the ALPHA phase II study (NCT03695510) involving advanced head and neck squamous cell carcinoma patients, the combination of Pembrolizumab plus Afatinib led to an improvement in ORR (17). This research direction holds promise for examining the combinatorial effect of Afatinib and pembrolizumab, offering potential for more personalized treatment in the future.
In the daily Afatinib setting, the LL6 trial (involving Southeast Asians, South Koreans, and Chinese) showed that 36% of patients experienced grade ≥ 3 adverse events (AEs), with diarrhea (5%), rash/acne (15%), and stomatitis/mucositis (5%) being common (7). Cumulative dose-related side effects could impact patient adherence. However, in our case, the weekly Afatinib strategy resulted in no Grade 3 AEs. Afatinib-related symptoms on nails and skin were effectively managed with antibiotics and topical steroids. A study by Daniel B. Costa et al. (2016) evaluating toxicity and response to pulsed Afatinib (280 mg once weekly) also found no grade 0 rash, grade 0 - 1 diarrhea, and no serious adverse events in the patient group (8).
Despite the stable status observed during the 6-month treatment with Afatinib, the consideration of cumulative dose-related side effects and the heterogeneous characteristics of the tumor, which may develop further resistance, raised questions regarding lesion resection. Previous studies on the combination of Afatinib and treatment primarily focused on patients at locally advanced stages, comparing Afatinib to classic neoadjuvant and adjuvant chemotherapy. To determine the surgical indication, dramatic clinical downstaging recorded by radiologic response, metabolic response, or histopathologic regression can be utilized. The Phase II ASCENT trial aimed to clarify the role of Afatinib in neoadjuvant settings among stage III NSCLC patients. Among the participants, 11 out of 19 patients (58%) achieved a response to Afatinib, with one initially deemed inoperable patient becoming a surgical candidate (9).
Following a real-world study review by Chieh-Lung Chen, perioperative TKIs were predominantly used in elderly patients (> 65 years old), with 5 out of 21 cases treated with Afatinib. This study also provided evidence that perioperative EGFR TKIs improved PFS in stage III NSCLC patients (18). To our knowledge, the indication of surgery at stage IV after a response to weekly Afatinib has not been reported. The low risk and low grade of side effects in the pulse Afatinib strategy did not affect the postoperative recovery and rehabilitation of this patient. Until July 2023, at the most recent examination, the patient had not shown any sign of disease progression since the lobectomy was performed, with no side effects of Afatinib, and an ECOG performance status of 0. In other words, the PFS was 16 months. In comparison to the two locally advanced cases taking Afatinib as the neoadjuvant reported by Mazzoni et al., the disease-free survival (DFS) after surgery was significantly lower at 9 and 10 months, respectively (10). The clinical practice on this patient supported the hypothesis that Afatinib had a good response on NSCLC patients with rare EGFR G719X mutation and showed the benefit of the pulse Afatinib strategy (weekly) in reducing the toxicity of this drug and improving patient compliance. It raises the question of whether we should indicate surgical intervention in delicate cases like ours. Our findings contributed more evidence in proving that EGFR G719C is sensitive to Afatinib and supporting the strategy of Afatinib with fewer adverse events than the classic daily dose. Our case also suggested the strategy of collaboration between medical oncologists and surgeons in treating NSCLC at an advanced stage.