1. Background
Cervical cancer is the fourth most common cause of cancer incidence and mortality in women with a rate of 570,000 new cases and over 300,000 deaths globally (1).
Along with the extensive use of successful screening programs in developed countries, the majority of patients with cervical cancer are detected at an early stage and can be completely cured by radical surgery or radiotherapy (2). However, in developing nations, over 80% of patients with cervical cancer are recognized in the locally advanced stage which will be treated with definitive chemoradiotherapy (3, 4). Almost one-third of patients who undergo definitive chemoradiotherapy experience cancer recurrence, typically within 2 years of completing treatment (5). After the first relapse, the prognosis becomes significantly poor (6). As a result, the accurate prediction of the treatment response is vital to promote patient survival who may profit from adjuvant therapy or intensive follow-up (7).
Personalized medicine based on different biomarkers has been used to predict prognosis and treatment strategy in many cancers such as breast, colon, and prostate, improving clinical outcomes (8-10). Therefore, finding nonaggressive pretreatment tools that help clinicians evaluate the heterogenicity of tumoral cell response to curative treatment, may lead to selecting an appropriate approach for each patient (11).
Unlike early-stage cancers which are mostly treated with radical hysterectomy, the absence of powerful predictive pathological or hematological biomarkers is felt in locally advanced patients with cervical cancer who undergo chemoradiotherapy. On the other hand, prospective studies have shown that PET scans can predict survival and recurrence-free survival 3 months after the end of treatment. Still, false positives and false negatives are reported in 39% of patients (12, 13).
Several cancers including cervical cancer progress from sites of infection (like the HPV virus) , persistent inflammation, and irritation. Inflammation affects every phase of tumor growth, from cancer initiation to promotion, proliferation, and survival (14). Accumulating research revealed that the inflammatory cells such as neutrophils and platelets in the ecosystem of tumors play a vital role in tumor progression. These parameters reduce adaptive immune system activity and induce the reproduction, progression, and spreading of cancer cells, which can cause resistance to radiotherapy and chemotherapy drugs (15).
Klopp and Eifel reviewed several biological factors that predict radiation response in patients with cervical cancer. The authors distinguished an assortment of bioclinical and morphological indicators, such as non-molecular biomarkers like hemoglobin (Hb) level, vascularity index, oxygen level, interstitial oncotic pressure, and PET SUV mean. This study also discussed the role of some molecular biomarkers like single-gene or multigene biomarkers and HPV species (16).
The relationship between various hematological parameters that indicate systemic inflammation such as neutrophil, lymphocyte, platelet count and Hb with the prognosis of cancers or treatment response has been investigated (17, 18). Among the diversity of parameters, absolute platelet to lymphocyte ratios (PLR) and neutrophil to lymphocyte (NLR), which reveal the balance between inflammatory induction of tumoral cells and anti-tumoral host response, could turn these ratios into a beneficial index to anticipate the cancer prognosis (19).
High NLR has been linked to regrettable outcomes, such as biliary tract cancers, breast cancer, gastrointestinal cancer, head and neck squamous cell carcinoma, soft tissue sarcoma, ovarian cancer, and colorectal cancer (20-28).
Regarding the limited data obtained from previous studies, these parameters are associated with advanced tumor stages, distant metastases, and poor survival in patients having cervical cancer. (29-31). There is limited research on the predictive value of these hematological parameters in response to definitive chemoradiotherapy in cervical cancer, and the results are controversial (32). This may be due to the retrospective nature of these literature with their inherent limits. A higher level of evidence studies is required to obviate issues like selection bias and control confounding factors.
A factor with acceptable specificity and sensitivity that individually or in combination with other risk factors can predict treatment response faster than conventional imaging, may help to intensify the treatment protocol on time and improve the outcome (26).
2. Objectives
To the best of our knowledge, this is probably one of the first prospective studies to specifically study the predictive value of pretreatment hematologic parameters in locally advanced cervical cancer who received chemoradiotherapy, which tries to remove confounding parameters that are seen in previous retrospective articles.
3. Methods
3.1. Study Protocol and Population
In this prospective cohort study, LACC patients who were treated with definitive chemoradiotherapy in the radiotherapy Oncology Clinic, at Shohadaye Haftom-e-Tir and Firozgar Hospitals (Tehran-Iran) from April 2021 to December 2022, were included. The Institutional Ethics Committee of Iran University of Medical Science approved this study (IR.IUMS.FMD.REC.1400.158). Both verbal and documented informed consent were also obtained. The article was run under the Declaration of Helsinki Guideline.
To determine the sample size, Mizunuma et al article (33) was used By using Fisher’s formula with an alpha error of 5%, and type 2 Error (beta) of 10%, proportion P1 = 0.81 and proportion P2 = 0.23 based on the mentioned study, a sum of 34 patients with LACC participated in this study. All patients underwent clinical staging by a complete physical exam and medical history. Pelvic MRI to determine Tumor and Nodal stage, and chest and abdominopelvic CT scan to assess Metastasis were done by a relevant oncologist. If any sign of bladder or rectal invasion was observed in MRI, sigmoidoscopy, or cystoscopy, was also carried out.
Inclusion criteria included patients who were in stage IB-IVA of cervical cancer as confirmed by gynecologists, biopsy tissue, and imaging studies and who were candidates for Definitive Chemoradiotherapy treatment. Patients who were candidates for adjuvant radiotherapy after total hysterectomy, patients who received chemotherapy (either induction or adjuvant), hormone therapy, or abdominopelvic radiotherapy before the current treatment, patients with specific inflammatory diseases such as IBD, rheumatic disease, acute or chronic infections, etc., as well as patients taking anti-inflammatory drugs such as corticosteroids and NSAIDs and patients who had incomplete radiation therapy course or did not take the routine follow up, excluded from the study.
The detailed information checklist regarding age, habitual and past medical history, and histological type of tumor based on histopathological features was confirmed by immunohistochemistry, tumor grade based on cellular differentiation on American College of Pathology system, stage by International Federation of Gynecology and Obstetrics (FIGO 2018), tumor size by greatest dimension on centimeter, pretreatment complete blood count (CBC) report, treatment response as reported in response evaluation criteria in solid tumor (RECIST 1.1), were registered. To protect the patient's information and ensure its confidentiality, the patients were coded, and the follow-up process was carried out with this code.
3.2. Treatment Schedule and Response Evaluation
To assess the pretreatment hematological parameter, we took CBC. In addition, two slides of peripheral blood smear (PBS) from each participant were taken before Radiotherapy. Taking PBS was postponed If the participant had signs of any infection, until full recovery. We noticed the patients did not smoke, heavily exercise, or take anti-inflammatory drugs at least 24 hours before taking PBS. The blood smears were analyzed by a skilled pathologist to reduce the laboratory error. Recorded data included absolute neutrophil count, absolute lymphocyte count, absolute platelet count, and Hb level. The definition of NLR was the absolute neutrophil count divided by the absolute lymphocyte count. PLR was the absolute platelet count divided by the absolute lymphocyte count.
Every patient received the combination of definitive external beam radiation therapy (EBRT) with a 3-dimensional conformal technique with weekly intravenous 40 mg/m2 cisplatin (CDDP) for 5 weeks. The target volume containing the Gross tumor, cervix, uterus, parametria, and vagina depends on the extent of involvement. The pelvic Lymph node such as common, external, and internal iliac and presacral Lymph nodes ± Inguinal/paraaortic LN is also included in the treatment volume. The Whole pelvic EBRT consists of 45 Gy in 25 fractions using 10 – 15 MV photons in 4 field techniques. At the end of EBRT 3-Dimensional high dose ratio (HDR), brachytherapy (with Cobalt 60) was performed weekly. A dose of 25 – 30 Gy in 4 - 5 separate fractions was given to the cervix and parametrial extension was observed in imaging. Every week during the chemoradiotherapy, the patient was assessed by history and physical examination to evaluate the adverse events of therapy and her compliance to complete the chemoradiotherapy and brachytherapy.
Three months after the completion of the chemoradiotherapy, the patient was examined by a gynecologic oncologist and underwent a Pelvic MRI and CT scan of the other sites if indicated. Patients with no evidence of the disease either in the examination or in the imaging findings, were classified as clinical complete response (CR). A residual tumor or the appearance of a new lesion during follow-up imaging or examination, was considered Non-complete response (Non-CR). After collecting and recording the information, the pretreatment hematological parameters, NLR, and PLR were analyzed and compared between two groups of responders.
3.3. Data Analysis
SPSS software (statistical package for social sciences, Chicago, IL) version 26 was used for statistical analysis. Frequency (%) was used for the preliminary report of qualitative variables. For quantitative variables, mean, standard deviation, and median were used. The normality was analyzed using the Kolmogorov Smirnoff (K-S) test. For comparison of qualitative parameters, the chi-square test was used. If the sample size in any subgroups was small, Fisher’s exact tests were applied. For the quantitative variable, if normality was established, an independent sample t-test was utilized otherwise the equivalent non-parametric statistical test (Mann-Whitney U test) was conducted. We performed receiver operating characteristic (ROC) curve analysis to assess the predictive value of the pre-hematological parameters in tumor response to definitive CRT and achieve a cut-off point with maximum sensitivity and specificity to determine patients who are at high risk for treatment failure based on hematological markers. For all the analyses, the 2-sided P-value statistical significance level was 0.05.
4. Results
4.1. Patient Characteristics
The sum of 34 eligible patients who were referred to the mentioned hospitals, were evaluated in this study. The mean age of the patients was 54.2 years with a range between 34 to 75 years old. The average tumor size was 5.41± 1.52 cm.
According to the FIGO 2018 staging system, the most common stage among patients was stage IIIC (LN involved) and IIB with the frequency of 13 (38.2%) and 12 (35.3%) patients, respectively. The other clinical tumoral features and pretreatment hematological parameters are presented in Table 1.
| Variables | Patients |
|---|---|
| Number | 34 |
| Age (y) | 54.2 ± 11.26 |
| Tumor histology | |
| Squamous cell carcinoma | 32(94.1) |
| Adenocarcinoma | 2(5.9) |
| Tumor grade | |
| Well differentiated | 12(35.3) |
| Moderate differentiated | 8(23.5) |
| Poor differentiated | 9(26.5) |
| Unknown | 5(14.7) |
| Tumor size (cm) | 5.41 ± 1.52 |
| Tumor size (cm) | |
| ≤ 2 | 1(2.9) |
| 2.1 - 5 | 15(44.1) |
| > 5 | 15(44.1) |
| Unknown | 3(8.9) |
| Stage | |
| Early (I-II) | 14 |
| Advance (III-IV) | 20 |
| Mean pre-hematological parameter ± SD | |
| Hemoglobin (mg/dL) | 11.33 ± 2.00 |
| White blood cell (×103/μL) | 7928.00 ± 4021.66 |
| Neutrophil (×103/μL) | 5158.65 ± 3159.70 |
| Lymphocyte (×103/μL) | 2132.77 ± 1068.31 |
| Platelet (×103/μL) | 282538.46 ± 77688.72 |
| Pre-NLR | 2.56 ± 1.31 |
| Pre-PLR | 161.04 ± 93.12 |
Abbreviations: NLR, neutrophil to lymphocyte ratio; PLR: Platelet to lymphocyte ratio.
aHistology, based on histopathologic features confirmed by Immunohistochemistry; Grade, based on the extent of cellular differentiation based on WHO2021; Stage, based on the International Federation of Gynecology and Obstetrics (FIGO stage) of 2018.
b Values are expressed as No. (%) or mean ± SD.
4.2. Comparison Between Two Groups of Responders
Out of 34 patients, 25 (73.5%) had complete responses and the remaining 9 (26.5%) had non-complete responses. The statistical comparative analysis of tumor characteristics and hematological parameters in 2 groups of CR and non-complete response is described in Table 2.
| Variables | Tumor Response to Treatment | P-Value | |
|---|---|---|---|
| Complete Response (N: 25) | Non-complete Response (N: 9) | ||
| Tumor histology | 0.47 | ||
| Squamous cell carcinoma | 24 (96) | 8 (88.9) | |
| Adenocarcinoma | 1 (4) | 1 (11.1) | |
| Tumor grade | 0.047 | ||
| Well differentiated | 10 (40) | 2 (22.2) | |
| Moderate differentiated | 8 (32) | 2 (22.2) | |
| Poor differentiated | 6 (24) | 3 (33.4) | |
| Unknown | 3 (12) | 2 (22.2) | |
| Stage | 0.81 | ||
| Early (I - II) | 10 (40) | 4 (44.4) | |
| Advance (III - IV) | 15 (60) | 5 (55.6) | |
| Tumor size | 5.25 ± 1.23 | 5.87 ± 2.1 | 0.46 |
| Age | 55.92 ± 9.2 | 53.03 ± 12.6 | 0.58 |
| Hemoglobin | 11.9 ± 1.78 | 9.46 ± 1.92 | 0.032 |
| WBC | 7.126 ± 5.403 | 10.464 ± 2.744 | 0.025 |
| Neutrophil | 4.32 ± 4.041 | 7.58 ± 2.231 | 0.001 |
| Lymphocyte | 2.231 ± 1.029 | 2.098 ± 1.003 | 0.89 |
| Platelets | 281.952 ± 95.092 | 286.22 ± 98.257 | 0.85 |
| NLR | 2.34 ± 1.36 | 3.35 ± 1.02 | 0.048 |
| PLR | 149.81 ± 53.66 | 164.41 ± 102.7 | 0.52 |
z Abbreviations: NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio.
a Histology, based on histopathologic features confirmed by Immunohistochemistry; Grade, based on the extent of cellular differentiation based on ACP; Stage, based on the International Federation of Gynecology and Obstetrics (FIGO stage) of 2018; Response, based on RECIST1.1. The prevalence of well-differentiated tumors in patients who completely responded to treatment was significantly higher than that of patients with non-complete responded to treatment (P = 0.047).
b Values are expressed as No. (%) or mean ± SD.
The non-complete responders showed higher pre-treatment leukocyte and neutrophil counts, NLR, and PLR than those of the complete responders. The pretreatment Hb and Lymphocyte in the non-complete responder was lower than those of the other group, but the statistically significant difference was only seen in Hb. The mean values of neutrophil, NLR, Hb, leukocyte, and PLR were 7.58 ± 2.231, 3.35 ± 1.02, 9.46 ± 1.92,10.464 ± 2.744 and 164.41 ± 102.7 in the non-complete responders as compared to 4.32 ± 4.041, 2.34 ± 1.36, 11.9 ± 1.78, 7.12 6 ± 5.403 and 149.81 ± 53.66 in the complete responder’s group, with a statistically significant P-value of 0.001, 0.048, 0.032 and 0.025, accordingly. However, no significant P-value was observed in the PLR parameter (P = 0.52).
4.3. Hematological Parameters Cut-off Point
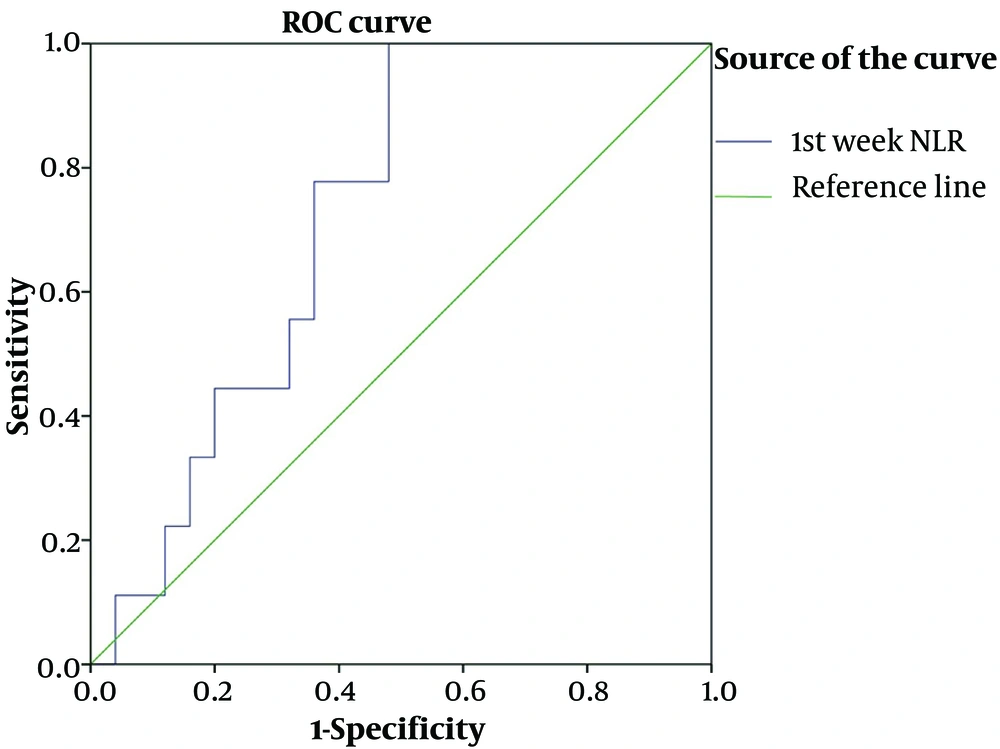
Receiver operating characteristic curve analysis was done to calculate the optimized cut-off point of pre-treatment NLR, PLR, and Hb for this prospective cohort study. The NLR cut-off value was 2.1 with AUC, 0.75; 95%; confidence interval (CI), 0.57 - 0.89 and P-value of 0.043 with sensitivity and specificity of 80% and 64%, respectively (Figure 1).
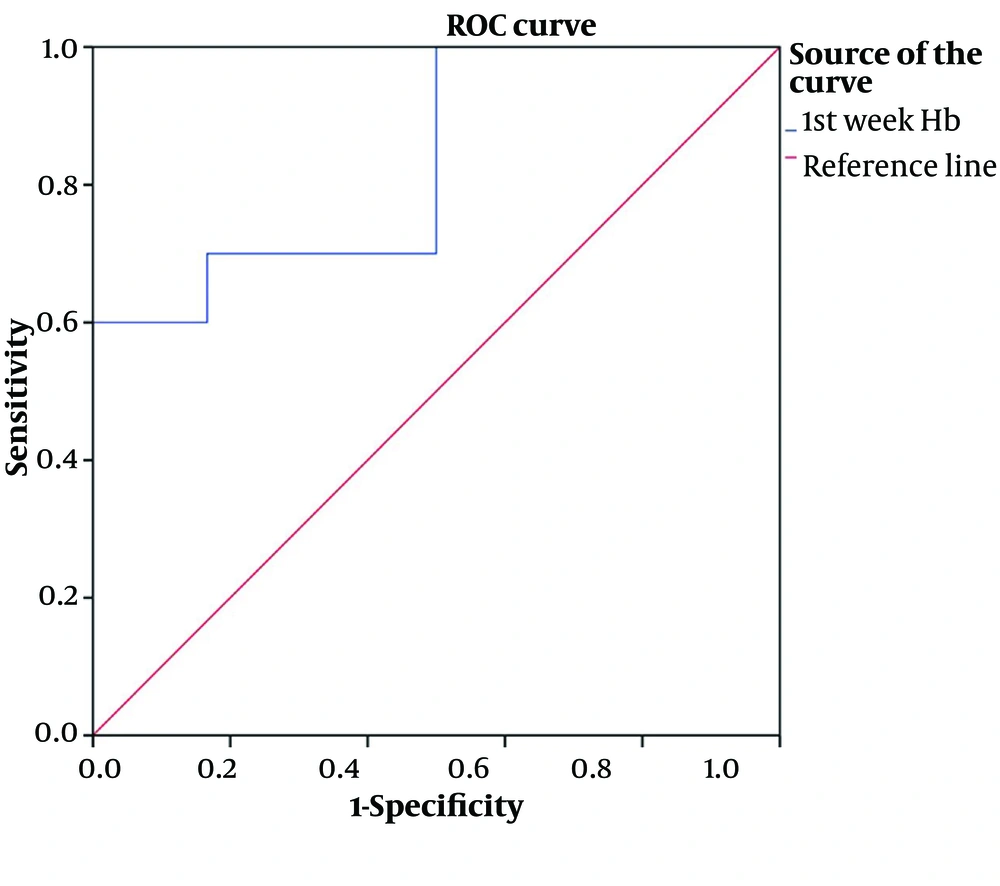
The cut-off value of Hb was 11 with an AUC of 0.833.; 95%; CI, 0.66 – 1.00 and P-value of 0.015 with sensitivity and specificity of 70% and 83%, respectively (Figure 2).
No significant relationship was observed based on the pre-treatment PLR cut-off point to predict response to treatment. [cut point, 154; AUC, 0.51, 95%; CI, (0.23 - 0.78); P-value, 0.91]
4.4. Comparison Based on Neutrophil-to-Lymphocyte Ratio Cut-off Point
The clinical characteristics of the tumor by the pre-treatment NLR cut-off value are shown in Table 3. As seen in this table, patients with NLR ≥ 2.1 had an advanced tumor in terms of tumor size and tumor stage but only in the tumor size item, the difference between the 2 groups is statistically significant with a P-value of 0.036. The difference between the other hematological parameters based on the NLR cut point is also shown in Table 3.
| Variables | 1st Week NLR | P-Value | |
|---|---|---|---|
| ≤ 2.1 (N: 17) | > 2.1 (N: 17) | ||
| FIGO stage | |||
| I, II. early | 9 | 5 | 0.253 |
| III, IV. advance | 8 | 12 | 0.418 |
| Age (y) | 55.29 ± 10.48 | 53.18 ± 12.22 | 0.59 |
| Tumor grade | 0.23 | ||
| Well differentiated | 8 (47.1) | 7 (41.2) | |
| Moderate differentiated | 5 (29.4) | 5 (29.4) | |
| Poor differentiated | 4 (23.5) | 5 (29.4) | |
| Tumor size | 4.89 ± 1.31 | 6.1 ± 1.56 | 0.036 |
| Hemoglobin | 12.22 ± 1.59 | 10.45 ± 1.87 | 0.007 |
| WBC | 5.700 ± 1.778 | 9.431 ± 4.230 | 0.004 |
| Neutrophil | 3.009 ± 1.019 | 6.674 ± 3.052 | 0.001 |
| Lymphocyte | 2.215 ± 1.091 | 1.955 ± 0.942 | 0.48 |
| Platelets | 236.733 ± 72.589 | 325.294 ± 93.633 | 0.007 |
| Complete response | 15 | 10 | 0.023 |
| Non-complete response | 2 | 7 | |
z Abbreviations: NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio.
a Stage, based on the International Federation of Gynecology and Obstetrics (FIGO stage) of 2018; Response: Based on Response Evaluation Criteria in solid tumor (RECIST1.1); The cut-off value is based on the Receiver operating characteristic (ROC) analysis.
b Values are expressed as No. (%) or mean ± SD.
5. Discussion
In the present study, we showed that in our Iranian patients mean values of leukocyte, neutrophil, and NLR levels before treatment in the non-complete responders to chemoradiotherapy were significantly higher than those of the patients with CR. Non-complete responders had higher PLR and platelet counts compared with those of the CR, but our data showed no statistically significant difference between the 2 groups. Conversely, non-complete responders had significantly lower Hb levels. These data proposed that there was a difference in inflammatory status between patients who responded completely and the group that did not respond to chemoradiation, and it is possible that persistent and progressive inflammation in non-complete responders could lead to poor oncologic outcomes. Hematological parameter such as high NLR is known as a poor prognostic factor in various cancers such as esophageal, colorectal, pancreatic, prostate, hepatic, biliary tract, breast, gastric, and lung cancers (27, 34, 35). But for the predictive value of this parameter in response to the definitive treatment, data is few (33, 36). As seen in our study, some previous studies have shown a similar relationship between pre-treatment hematological parameters and response to therapy in cervical cancer. In a study published by Gavrilescu et al., there was a significant relationship between NLR and MNM (neutrophil multiply monocyte) and lymph node metastasis, parameter involvement, LVI, and tumor size in patients with cervical cancer. NLR and MNM were significantly lower in patients who achieved complete CR response with neoadjuvant treatment (37). Also, in a study by Cho et al, patients who had tumor-related leukocytosis (in the form of 2 or more leukocytosis above 9000 at the beginning or during treatment), were significantly younger, had a larger tumor size, more advanced stage with high percentage of lymph node metastasis. People who experienced leukocytosis and neutrophilia were significantly more resistant to radiotherapy and had less CR (38).
Although the role of NLR and PLR has been evaluated in several studies, the cut-off point varies widely in different studies and different cancers. In our study, the optimal threshold by ROC curve analysis for predicting response to treatment was 2.1 for NLR and 11 for Hb. A similar retrospective study was conducted by Chauhan et al. In this study, out of 90 patients examined, 60 patients had a CR to chemoradiotherapy. The average number of platelets, NLR, and PLR in patients with cervical cancer who did not respond to chemoradiotherapy treatment was significantly higher. Receiver operating characteristic curve analysis showed that the cut-off Hb was 11, platelets 177000, NLR 3, and PLR 70 (39). Also, Mizunuma et al reported similar findings in Japan. They divided 56 patients with cervical cancer into two groups with NLR >2.5 and NLR < 2.5 based on the average NLR. Patients with NLR lower than 2.5 had a significantly better response to radiation therapy (81% vs. 22.9%) and patients with NLR > 2.5 had more advanced tumors in terms of size and stage and lymph node involvement. This group also had shorter OS and PFS (33). The pretreatment PLR and NLR median points were 133.02 (interval 36.3 - 518.16) and 3.03 (interval 1.04 - 13.03), respectively, in the Onal et al. study. Both high Pretreatment NLR and PLR above the median cut-off point were related to metastatic lymph nodes, larger tumor size, and less oncologic responses to definitive radiotherapy (40).
No cut-off point was identified for platelets and PLR in our study. However, in Lopes et al.'s study, increased platelets were not considered as a prognostic factor in cervical cancer (41). In a study on stage IIB cervical cancer patients, NLR, and MLR were better predictors than PLR and BLR in prognosis and recurrence risk. NLR was also a potential marker for treatment response. However, PLR was evaluated as a poor indicator for predicting response to treatment (42).
Pretreatment NLR above 2.1 was significantly associated with larger tumor size, high leukocyte, high neutrophil, low Hb, and high platelet. Although the patients with advanced stage (stage III & IV) had a higher NLR compared to that of the early stage (stage I & II), the difference was not significant. These results confirm that the tumor with high inflammatory indices can also have more advanced tumoral characteristics, which can lead to poor treatment outcomes besides the inherent characteristic of these inflammatory parameters.
The results of our study were consistent with many studies (29, 43, 44). However, the absence of some correlations such as platelets or PLR with response to treatment, as well as the lack of correlation of some clinicopathological characters of the tumor with NLR or PLR, may be due to the limited sample size and unicentric setting. Another limitation of our study is that the inclusion criteria were only the evaluation of the response of patients who underwent definitive chemoradiotherapy, and these results cannot be extended to other treatment modalities (such as radical surgery, chemotherapy, and immunotherapy…).
According to our knowledge, this study was the first prospective study in this field and Iran Country. Some of the limitations of previous studies, which have an impact on the outcome, were eliminated as much as possible; among them, due to the prospective nature of the study, only patients who did not have inflammatory diseases, acute infection, metabolic syndromes, rheumatic diseases, or did not take drugs affecting the inflammation process, were included in the study, and confounding factors such as smoking and heavy exercise up to 24 hours before sampling were excluded. All patients underwent the same examination, treatment, and follow-up. One of the other important points is that the method of collecting the samples was uniform and only one expert pathologist examined all blood smears of the patients manually before treatment. This phenomenon reduced laboratory error, which could not be done in other studies due to their retrospective nature.
According to the above results, hematological parameters like NLR can be used as a cheap and accessible inflammatory index to predict the response to treatment. On the other hand, these inflammatory markers can guide choosing the optimal and probably more intensified approach for this high-risk patient at the right time. For example, on the one hand, increasing the Hb level above 11 before treatment may improve the response, on the other hand, patients with high NLR may need dose escalation in both chemotherapy and radiotherapy schedules. It is a possible hypothesis that the rise of these inflammatory factors occurs even earlier than the imaging findings and tumor markers changes during relapse. More prospective studies with a large sample size should be conducted to assess CBC of patients not only before the treatment but also during the treatment and at the end of the therapy (45). This could lead to the validation of these hematological factors not only as a predictive factor but also as a follow-up marker for patients with cancer.