1. Background
Endometrial cancer (EC) holds the position of the most prevalent gynecological malignancy in Western Europe and the United States (1), with an estimated lifetime risk of 2.6% for women residing in developed nations (2). According to the cancer statistics in Iran, the 5-year prevalence of breast cancer was about 56,000 patients in 2020, and its age was, unfortunately, decreasing (3). The overall 1, 5, 10, 15, 20, and 25-year survival rates of breast cancer were 95%, 75%, 60%, 47%, 46%, and 46%, respectively (4). GLOBOCAN 2020 reported breast cancer as the new most common cancer, surpassing lung cancer, with an estimated 2.3 million new cases (11.7%) (5). Its overall incidence was from 2-fold to 3-fold higher in transitioned versus transitioning countries. Death rates from breast and cervical cancers remain higher in developing countries. The global cancer burden is projected to rise 47% by 2040, with a steeper increase in developing countries (64% - 95%) compared to developed nations (32% - 56%) (6). More specifically, the age-adjusted incidence rate rose by 0.69% between 1990 and 2019 (7). This increase was particularly marked in select nations, including Italy, Saudi Arabia, and Singapore, where the age-adjusted incidence rates surged by more than 3% between 1990 and 2019 (8).
Endometrial carcinomas arise in the innermost layer of the uterus and account for more than 90% of primary malignancies of the uterine corpus. These cancers are categorized into 2 distinct types based on clinical and pathological features (9). In patients with low- and medium-risk EC, the most crucial prognostic indicators for disease progression encompass lymphovascular involvement, depth of myometrial invasion, tumor size exceeding 2 centimeters, involvement of the distal uterine segment, and clear cell or serous carcinoma histology (10, 11). Enlarged adipose tissue mass and conditions associated with metabolic syndrome, such as diabetes and polycystic ovary syndrome, are well-established risk factors for developing EC (12).
Although several contributing factors to EC have been recognized, the exact cause of the disease remains largely uncertain (13). Nevertheless, the transition from the pre-cancerous state of intraendometrial neoplasia to endometrioid carcinoma appears to be well-documented (14). Establishing the risk factors that contribute to the onset and progression of this disease remains a significant scientific pursuit. This knowledge could pave the way for more effective treatment strategies for high-risk subgroups and mitigate the risk of overtreatment in the low-risk group (15). In line with this pursuit, a previous similar study conducted at a university tertiary hospital in the United States revealed a 5-year overall survival (OS) rate of 62.5% and a disease-free survival (DFS) rate of 46.9% (5).
2. Objectives
Inspired by these findings, this study aimed at evaluating the OS and DFS of EC patients in a monocenter in Shiraz, Iran, and identifying factors that influence their prognosis.
3. Methods
This retrospective cohort study investigated the medical records of all patients with definitively identified uterine EC, as confirmed by pathological analysis, who underwent treatment at Motahari Tumor Clinic in Shiraz, Iran, between 2014 and 2018. The study adhered to the principles of the Declaration of Helsinki, and the protocol was approved by the Research Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1402.136).
The study required demographic data (age, marital status), pathological characteristics at diagnosis (cervix involvement, adnex involvement, lymph node involvement, number of deliveries, tumor grade, International Federation of Gynecology and Obstetrics (FIGO) stage, histological types, myometrial invasion, lymphovascular invasion (LVI), peritoneal cytology), second treatment type, and postoperative and outcome data (reoccurrence, latency period to reappearance if applicable, radiation therapy, chemotherapy, duration of observation, and clinical status after follow-up) from the patients' medical records. All patients received the same initial treatment. To obtain the required information on survival and recurrence, phone calls were made to the patients. The patients' survival status at the time of the study was determined by reviewing medical documentation (if the patient had visited a doctor within the past 6 months) or by contacting the patient or a family member by phone. Given that the study was conducted over 7 years, the DFS and OS were calculated in the 1, 3, and 5-year periods following treatment completion.
Tumor staging was determined based on the revised classification system established by the FIGO 2008 (16). Histological typing adhered to the criteria outlined by the World Health Organization (17). The survival variables that capture both the time duration and the occurrence of an event were defined as follows.
1. For OS analysis, the time component was defined as the period from primary surgery to the patient's death or until completion of the 7-year follow-up period, irrespective of disease recurrence.
2. For DFS analysis, the time component was defined as the interval following treatment, during which no signs of cancer were detected. This term can be applied to both individual patients and groups of individuals within a study cohort.
Inclusion criteria were the definitive diagnosis of primary EC. Exclusion criteria were incomplete or unavailable data in the patients' medical records and a lack of access to records pertaining to treatment outcomes.
3.1. Statistical Analysis
Descriptive features of patients were summarized as means ± standard deviations and frequency percentages. The probability of DFS and OS at 1, 3, and 5 years was estimated using the life table method. Due to the high correlation between various tumor characteristics and the limited number of events for certain factors, the use of Cox regression to estimate hazard ratios was not considered reliable for precise prognostic factor assessment. Instead, Kaplan-Meier survival analysis was employed to evaluate the impact of the studied clinical features on DFS. The life tables procedure was used to examine the probability distribution of DFS/OS times at 1, 3, and 5 years, while the Wilcoxon (Gehan) test was applied to compare survival distributions between categorical variables. Statistical analysis was conducted using SPSS 21.0 software, with a P-value of less than 0.05 considered statistically significant. The significance of curves by prognostic factors was determined using the log-rank test. Kaplan-Meier survivor function graphs were generated using STATA 17 software to visualize differences between groups.
4. Results
This study included 360 individuals with EC that 39 (10.8%) were experienced relapse. The estimated probability 1, 3, 5 years DFS were 93%, 90%, 88%, respectively, and OS were 92%, 89%, 89%, respectively (not shown in Tables).
Table 1 shows some important demographic and clinical distribution factors of patients with EC. The mean age ± SD of patients was 57.67 ± 7.94 years, and 97.2% were married. The mean number of deliveries was 2.74 ± 1.63. Invasiveness features showed involvement of cervix stroma (12.8%), myometrium (lower than 50% = 70%, more than 50% = 30%), LVI (1.9%), adenex (5.30%), and lymph node (1.7%). Endometrioid carcinoma was the predominant type, accounting for 93.3% of cases (334), followed by papillary serous carcinoma (5.6%) (n = 20). Clear cell carcinoma was relatively uncommon (n = 4, 1.1%). The observed relapse rate was 10.8% (n = 39).
| Variables | Values |
|---|---|
| Mean age (SD) | 57.67 ± 7.94 |
| Marital status | |
| Married | 350 (97.2) |
| Not married | 10 (2.8) |
| Cervix involvement | 46 (12.8) |
| Adenex involvement | 19 (5.30) |
| Lymph node involvement | 1 (1.7) |
| Mean number of deliveries | 2.74 ± 1.63 |
| Tumor grade | |
| Grade 1 | 278 (77.2) |
| Grade 2 | 47 (13.1) |
| Grade 3 | 35 (9.7) |
| FIGO stage | |
| Stage 1A | 284 (78.9) |
| Stage 1B | 31 (8.6) |
| Stage 2 | 31 (8.6) |
| Stage 3A | 7 (1.9) |
| Stage 3C | 5 (0.3) |
| Stage 3B | 0 (0) |
| Stage 4 | 6 (1.7) |
| Subtype | |
| Endometroid | 334 (93.3) |
| Papillary serous | 20 (5.6) |
| Clear cell carcinoma | 4 (1.1) |
| Myometrial invasion | |
| Lower than 50% | 252 (70) |
| More than 50% | 108 (30) |
| Lymph vascular invasion | |
| Absent | 353 (98.1) |
| Present | 7 (1.9) |
| Peritoneal cytology | |
| Negative | 356 (98.9) |
| Positive | 4 (1.1) |
| Second treatment | |
| None | 247 (68.6) |
| Chemo radiation | 21 (5.8) |
| Brachytherapy | 49 (13.6) |
| EBRT/brachytherapy | 3 (0.8) |
| Brachytherapy/ chemo radiation | 40 (11.10) |
Abbreviation: FIGO, International Federation of Gynecology and Obstetrics.
a Values are expressed as mean ± SD or No. (%).
At diagnosis, pathological grades 1, 2, and 3 were observed in 77.2%, 13.1%, and 9.7% of patients, respectively. Stage 1A, 1B, stage 2, stage 3A, stage 3C, and stage 4 were observed in 78.9%, 8.6%, 8.6%, 1.9%, 0.3%, and 1.7% of patients, respectively; stage 3B was not observed in our samples.
At diagnosis, 59 patients were grade 3 (29.5%), and 67 were stage 3 (n = 43, 21.5%) or IV (n = 24, 12.0%). The median and minimum, and maximum tumor sizes were 4.0 (> 0 - 12) cm.
Cytopathology was positive in 1.1% of patients, and 68.6% of patients needed no second treatment, 5.8% were treated using chemo radiation, 13.6% were treated with brachytherapy, 0.8% were treated with EBRT/brachytherapy, and 11.10% were treated with Brachytherapy/chemo radiation (Table 1).
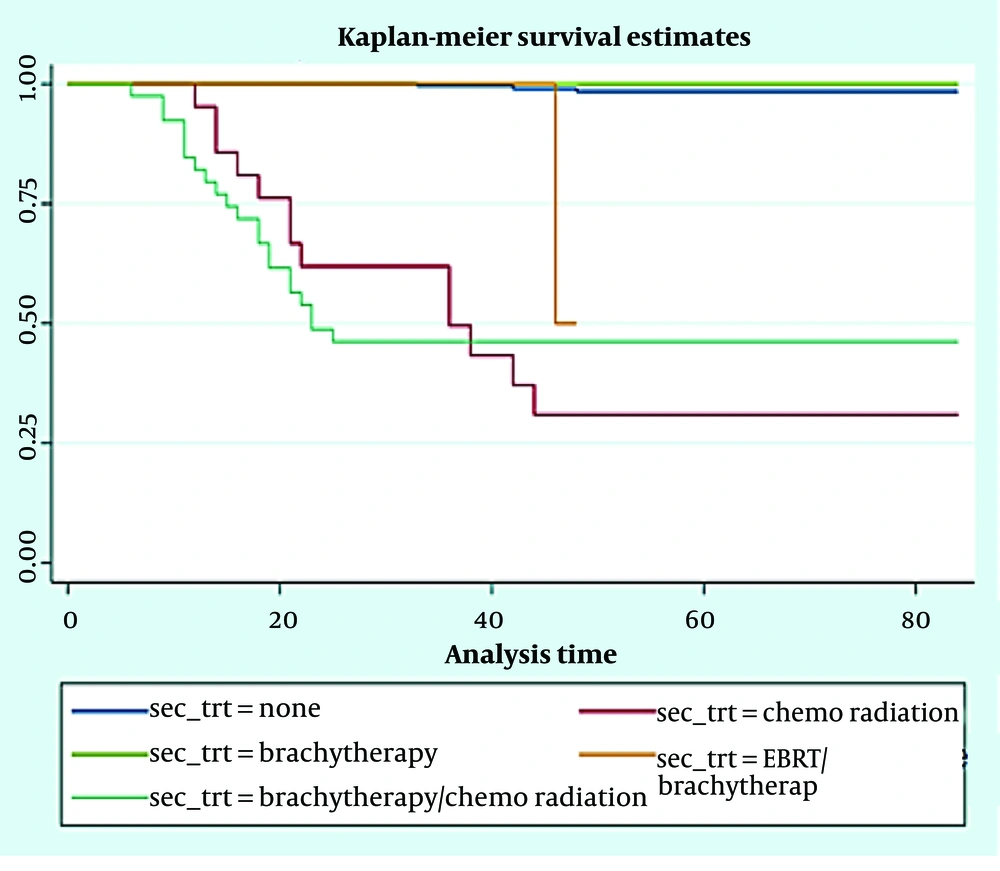
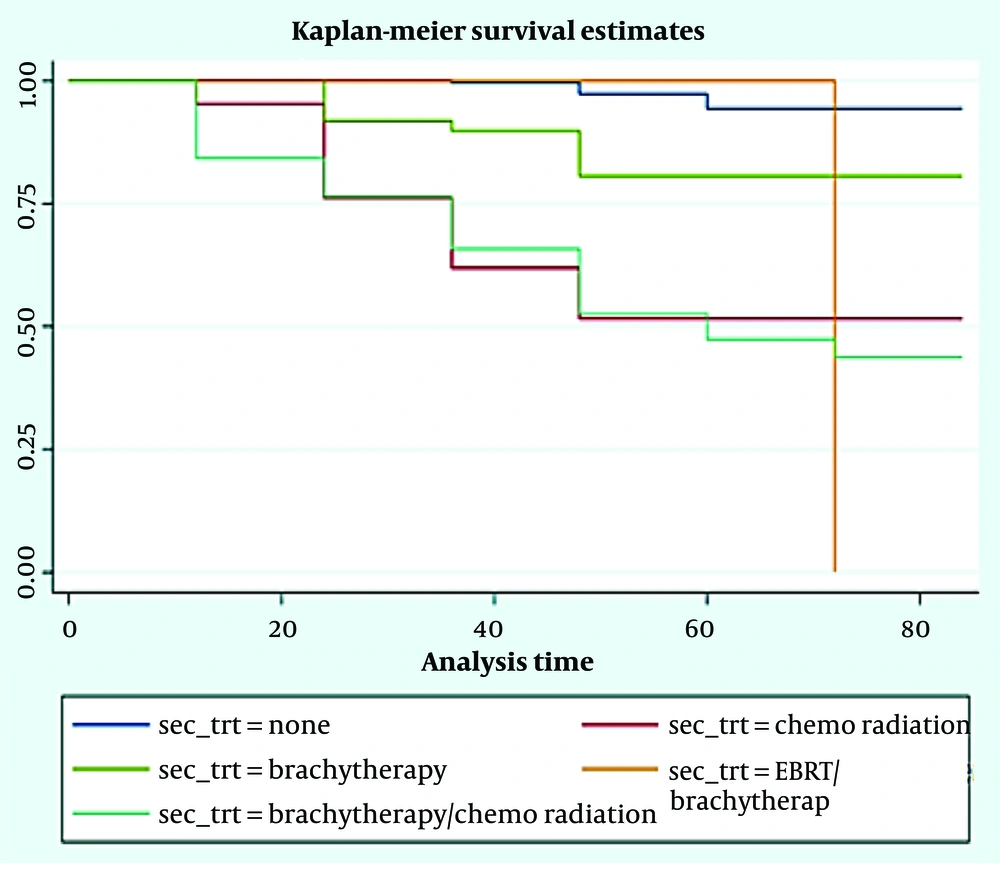
As shown in Table 2, prognostic variables significantly affected DFS and OS at 1-3-5 years included: The FIGO stages, tumor grade, marital status, lymph vascular invasion, deliveries, and age group of patients (for all of them, P-value was less than 0.001 except for AGE and Relapse-DFS). There was a significant difference among the studied patients in terms of DFS (Figure 1) and OS (Figure 2) at 1-3-5 years after receiving the second treatment (P < 0.001).
| Variables | Disease Free Survival (%) | P-Value a | OS (%) | P-Value a | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 1 | 3 | 5 | |||
| Deliveries | 73 | 73 | 73 | < 0.001 | 95 | 84 | 69 | 0.004 |
| 1 - 2 | 98 | 97 | 97 | 98 | 95 | 93 | ||
| 3 ≤ | 92 | 88 | 87 | 98 | 91 | 78 | ||
| Age (y) | 0.81 | < 0.001 | ||||||
| < 50 | 90 | 90 | 90 | 97 | 93 | 85 | ||
| 50 - 70 | 93 | 89 | 89 | 99 | 95 | 87 | ||
| 70 < | 87 | 87 | 87 | 87 | 52 | 24 | ||
| Marital status | < 0.001 | 0.05 | ||||||
| Married | 94 | 91 | 91 | 98 | 92 | 82 | ||
| Not married | 40 | 30 | 30 | 90 | 70 | 70 | ||
| FIGO Stage | < 0.001 | < 0.001 | ||||||
| Stage 1 | 96 | 93 | 93 | 99 | 96 | 87 | ||
| Stage 2 | 87 | 87 | 87 | 100 | 84 | 69 | ||
| Stage 3 - 4 | 0 | - | - | 57 | 14 | 14 | ||
| Tumor Grade | < 0.001 | < 0.001 | ||||||
| Grade 1 | 99 | 98 | 98 | 100 | 99 | 93 | ||
| Grade 2 | 85 | 76 | 72 | 96 | 71 | 55 | ||
| Grade 3 | 47 | 35 | 34 | 80 | 60 | 44 | ||
| Lymph vascular invasion | < 0.001 | < 0.001 | ||||||
| Absent | 93 | 90 | 90 | 98 | 93 | 83 | ||
| Present | 36 | 36 | 36 | 71 | 29 | 29 | ||
| Second treatment | < 0.001 | < 0.001 | ||||||
| None | 100 | 99 | 98 | 100 | 100 | 93 | ||
| Chemo radiation | 62 | 31 | 31 | 95 | 61 | 50 | ||
| Brachytherapy | 100 | 100 | 100 | 100 | 90 | 50 | ||
| EBRT/brachytherapy | 100 | 50 | - | 100 | 100 | 100 | ||
| Brachytherapy/chemoradiation | 49 | 46 | 46 | 80 | 62 | 44 | ||
Abbreviations: OS, overall survival; FIGO, International Federation of Gynecology and Obstetrics.
a Wilcoxon (Gehan) statistic
Figure 1 shows the DFS times based on the second treatment process for patients. Among patients, 1.2% with no second treatment, 6.1% with chemo radiation, 33.3% patients with EBRT/brachytherapy, and 55% of patients with brachytherapy/chemo radiation experienced relapse during the follow-up of the study. None of the patients treated with brachytherapy experienced relapse. Event-free survival times of the 5 groups were statistically significant (log rank statistics = 212.308, P < 0.001).
Figure 2 shows patients' disease OS times based on the second treatment process for patients. Among patients, 3.2% had no second treatment, 47.6% of patients were with chemo radiation, 18.4% with brachytherapy, 33.3% with EBRT/brachytherapy, and 57.5% of patients with Brachytherapy/chemo radiation experienced death during the follow-up of the study. The DFS times of the 5 groups were statistically significant (log rank statistics = 109.113, P < 0.001).
The prognosis for EC is generally favorable, with reported 5-year OS rates ranging from 80.0% to 91.6% and 5-year DFS in patients with FIGO IA stage disease is over 85% (18, 19).
5. Discussion
The EC is a prevalent malignancy among women, with irregular bleeding being the most common symptom (18). Given the variation in disease recurrence rates from 2.9% to 28.6% in early-stage EC (19, 20), this investigation particularly focused on identifying factors linked to reduced 1, 3, and 5-year DFS and OS of EC patients. In this study, the estimated probability of 1, 3, and 5-year DFS EC were 93%, 90%, and 88%, respectively, and OS were 92%, 89%, and 89%, respectively, which is similar to data reported by Ebring et al. (21), that 3-year DFS and OS were 81.5% and 83.1%, respectively.
To our knowledge, no studies have investigated 1- and 3-OS and DFS in the literature. However, available data suggest a range of 80.0% to 91.6% for 5-year OS, with a DFS exceeding 85% in patients diagnosed at FIGO stage IA (22, 23). Jeppesen et al. (23) further reported a 7% overall recurrence rate within 3 years, with a concerning 48.1% vaginal recurrence rate and a corresponding 5-year OS of 64.8%. Similarly, Gayar et al. observed an 8% recurrence rate in their study of early-stage endometrioid EC, and recurrence rates vary between 2.9% and 28.6% (24). A total of about 4% to 20% of the patients with EC develop a locoregional recurrence, mostly among patients with locally advanced disease (25). The previous study showed that in these patients, survival rate is thought to be related to site of relapse as the most important factor, but also disease-free interval, and postoperative treatment as independent prognostic variables (26).
In this sample of consecutive EC patients, endometrioid carcinoma was the prevalent histological subtype, accounting for approximately 93.3% of cases, while papillary serous carcinoma represented approximately 5.6% of cases. Clear cell carcinoma was relatively rare, accounting for only 1.1% of cases. This distribution aligns with international data indicating endometrioid carcinoma as the predominant subtype, representing over 85% to 90% of cases (27). It is noteworthy that there is an association between the histological subtype and the extent of the tumor at the time of diagnosis. For instance, endometrioid carcinoma was prevalent in stage 1A (78.9%), stage 1B (8.6%), stage 2 (8.6%), stage 3A (1.9%), and stage 3C (0.3%). Stage 4 cases were exclusively composed of endometrioid carcinoma (1.7%). In contrast, another study documented the prevalence of endometrioid carcinoma (82%), serous adenocarcinoma (5.4%), clear cell adenocarcinoma (2.2%), and mixed carcinoma (2.5%) (19). Notably, our cohort did not include any cases of mucinous adenocarcinoma, adenosquamous carcinoma, undifferentiated carcinoma, or mixed carcinoma.
In our study, as in previous studies (28), the FIGO staging system demonstrated a significant association with tumor recurrence and mortality. Patients with FIGO stage 1 cancer exhibited 96%, 93%, and 93% DFS rates at 1, 3, and 5 years, respectively, compared to 87% for all studied years in patients with FIGO stage 2, which was statistically significant. Consistent with data reported by Sasano (29), the FIGO staging system emerged as an independent indicator of survival. Patients with advanced disease exhibited a 4.95-fold elevated risk of mortality compared to those with stage 1 EC. In a study conducted by Bajracharya and Juan (30), they found that the stage of EC was one of the most important prognostic factors. In another study, Karateke et al. (31) found that the 5-year survival rates for patients with stage 1, 2, 3, and 4 EC were 83.3%, 80%, 62.5%, and 33.3%, respectively.
Additionally, in line with our findings, pooled data from the PORTEC 1 and PORTEC 2 studies demonstrated that patient age, tumor grade, and lymphovascular space invasion (LVSI) were robust predictors of OS probability (32). Patient age has consistently been identified as a prognostic factor for recurrence and survival in multiple research studies (33). Our study also revealed that older women had a less favorable prognosis, with survival rates declining with advancing age (P < 0.05). A comprehensive analysis of 165 women with EC demonstrated a strong association between advanced age and compromised survival (34). Similarly, Li et al. (35) established that EC survival diminishes in older patients. However, Karateke et al. (31) and Lin et al. (36) discovered that age at diagnosis was not a significant predictor of survival for EC. In most studies, histological grade is recognized as the most established factor for recurrence (5, 7). In our study, tumor grading emerged as a significant risk factor predictor associated with poor outcomes. The probability of DFS and OS at 1, 3, and 5 years decreased with increasing tumor grade. The detrimental effect of poorly differentiated tumors on survival was consistent across all grades. A study by Reisinger et al. (37) involving 51 patients with stage 2 EC revealed that tumor grade was the most significant predictor of survival. The study found that only 37% of patients with grade 3 tumors survived for 5 years. The prognostic significance of myometrial invasion depth and LVSI in predicting lymph node metastasis, tumor recurrence, and negatively impacting survival has been widely recognized in various studies (38, 39). Dos Reis et al. (40) proposed that if extensive LVSI is identified, even in patients classified as low-risk, lymph node dissection should be performed. Consistent with these findings, our study also revealed that the presence of LVSI was a statistically significant factor associated with reduced DFS and OS.
Concerning the impact of marital status on cancer-related prognosis, our findings revealed that married patients exhibited a survival advantage compared to unmarried patients in terms of DFS. Hence, unmarried patients diagnosed with endometrial carcinoma had an elevated risk of mortality. As highlighted in the literature, marital status emerges as a significant predictor associated with cancer diagnosis and prognosis across various malignancies, including liver cancer (41), gastric cancer (42), breast cancer (43), and ovarian cancer patients.
Our findings revealed a significant association between the number of deliveries and improved 1, 3, and 5-year DFS and OS in endometrial carcinoma patients. This finding aligns with previous research conducted by Alkbretsen et al. (44), who reported a favorable survival trend among parous women compared to nulliparous women. Notably, the protective effect of childbirth was most pronounced among women with the shortest interval between their last delivery and diagnosis. This intriguing pattern may be attributed to the elevated progesterone levels and interruption of continuous estrogen stimulation during pregnancy (45).
The contemporary approach to gynecological cancer treatment emphasizes the judicious use of adjuvant therapy in early-stage EC, given the favorable prognosis and the risk of overtreatment (46). In our study, the relapse rates were 1.2%, 6.1%, and 33.3% for patients without second treatment, chemoradiation, and EBRT/brachytherapy, respectively, while the death rates were 3.2%, 47.6%, 18.4%, 33.3%, and 57.5% for patients with the corresponding treatment modalities, respectively. Previous clinical trials suggest that adjuvant vaginal brachytherapy in early-stage; low-risk EC does not significantly impact long-term disease control (47). Although adjuvant radiotherapy has been shown to improve local control of the disease in stage 1 FIGO EC patients, it did not have a significant impact on OS (48). In contrast, a study by Jeans et al. (49) concluded that brachytherapy is a suitable treatment option for patients with negative peritoneal cytology and early-stage clear cell, serous, or mixed endometrial carcinoma. Shinde et al. (50) also discovered that adjuvant brachytherapy in FIGO IA EC patients with unfavorable histology significantly enhances OS.
5.1. Conclusions
This study found that the median 1, 3, and 5-year DFS were 93%, 90%, and 88%, respectively, and for OC were 92%, 89%, and 89%, respectively. The FIGO stages, tumor grade, marital status, lymph vascular invasion, number of deliveries, and age group of patients were identified as predictors of survival. Early detection of endometrioid EC enables optimal surgical intervention. The findings of our study may contribute to a better understanding of its clinical behavior. The findings of the current study revealed that adjuvant brachytherapy significantly extended the DFS and OS rates in patients with high-intermediate and high-risk EC.
5.2. Limitations
Since the study was retrospective, data on treatment-related toxicities were not systematically collected, which could limit the comprehensiveness of the findings. Moreover, the single-center design of the study raises concerns about the generalizability of the results, as the sample may be skewed towards patients with more advanced disease stages. Large-scale, nationwide studies encompassing multiple medical centers are essential to establish a more comprehensive understanding of patient survival and its determinants in EC patients. Additionally, it is important to note that the lack of an electronic GYN cancer registry at the participating cancer center was a limitation of this study.