1. Background
Meningioma is a prevalent form of tumor within the central nervous system (CNS), originating from arachnoid cap cells. It accounts for approximately 30% of all primary intracranial tumors in adults, and it is less common in children and adolescents (0.4 - 4.6%). The frequency of meningiomas is 83 cases per 100 000 individuals, with a greater predominance in females (with a sex ratio favoring females at 2-4:1). As age increases, the incidence of meningioma rises; the frequency of meningiomas in the 0 to 19 age group is 0.14/100 000, while it is 37.75/100 000 in the 75 to 84 age group. The incidence of meningioma is the lowest in African Americans (3.43 per 100 000 people) and highest among Whites (9.52 per 100 000 people) (1).
When it comes to treating meningiomas, surgeons typically aim to remove them as completely as possible, while ensuring that the healthy brain tissue surrounding the tumor is safeguarded during the operation. This procedure is designed to alleviate symptoms resulting from the tumor, alter its natural progression, and ultimately enhance the patient's overall quality of life (1).
When a craniectomy is undertaken, the regular control of intracranial pressure, cerebrospinal fluid (CSF) dynamics, and blood circulation within the brain is disturbed, following the Monro-Kellie hypothesis. This disruption can result in possible complications like the buildup of cerebrospinal fluid, hydrocephalus, and a phenomenon referred to as sunken flap syndrome or trephined syndrome (2).
Cranioplasty refers to a surgical procedure that aims to repair cranial defects that are either acquired or congenital in nature. The main goals of cranioplasty are to protect the brain, reconstruct any lost anatomical structures, and improve the overall aesthetics of the skull. This procedure may be indicated for a variety of reasons, including traumatic injuries, decompressive craniectomies, tumor removal, complications arising from prior cranioplasties, and congenital deformities (3).
Cranioplasty has a twofold function, acting as a protective shield for cerebral structures and as a therapeutic intervention to manage changes in CSF, blood circulation, and the brain's metabolic needs. Furthermore, it can offer aesthetic advantages by reconstructing the deformities in the cranial bone (4)
Various materials are available for this purpose, including the patient's bones (autograft). There are cranial bones alone or bones from other parts of a patient's body used for autologous cranioplasty, bones from other patients or cadavers (allograft), bones from animals (xenograft), for allograft and xenograft because of their increased incidence of infections, resorption and rejection they are no longer considered suitable for use in cranioplastic surgery or synthetic materials. Thanks to the advancement of computerized personalization and 3D printing, shorter operation times have been achieved as well as improved results in cosmetics. Frequently used synthetic materials are acrylic or titanium mesh (5).
Polymethylmethacrylate (PMMA) is an acrylic resin that can be shaped and, once solidified, offers a level of strength and protection similar to the natural skull. Nonetheless, liquid PMMA must be created and solidified in the operating room, which can complicate its usage and increase the likelihood of embedding small particles in the scalp. Due to its limited integration with adjacent tissue and a higher risk of displacement, its popularity has waned in recent years. Nonetheless, newer techniques for constructing cranial implants through computer-aided design and computer-aided manufacturing (CAD/CAM) technology generate a model of the skull defect along with a pre-made PMMA implant. Due to its notable safety record, prefabricated PMMA is gaining traction as the preferred implant material at some medical facilities and is also more cost-effective to produce than titanium mesh (6).
Computer-aided design and computer-aided manufacturing technology has revolutionized cranioplasty by enabling surgeons to perform one-step reconstructions with patient-specific implants (PSI), especially for large benign tumor resections without skin invasion. This technology permits the creation of a stereolithographic model (3D printed skull), and certain researchers have devised indirect approaches that include intermediary stages like shaping the PSI directly onto the 3D printed model, using PMMA. These techniques have substantially enhanced the precision and effectiveness of cranioplasty surgeries (7).
Meningiomas are the most common primary benign intracranial tumors in adults that arise from the meninges of the brain and spinal cord. While surgical resection is often the treatment of choice, cranial defects may result from such procedures, leading to significant functional and aesthetic impairments. In recent years, acrylic cranioplasty has emerged as a promising technique for repairing these defects.
2. Objectives
In this case series, we present a retrospective analysis of patients, who underwent acrylic cranioplasty for cranial defects following meningioma surgery, highlighting the efficacy and potential benefits of this approach.
3. Methods
A retrospective review was done on all patients, who experienced reconstructive cranioplasty after meningioma surgery at neurosurgical unit care between November 2021 and March 2023. Data were collected from post-surgery reports, and the study was approved by the local institutional review board (IRB) as an audit. Individual patient consent was not required since the study was retrospective in nature and used existing data.
The information analyzed for this study includes patient age and sex, indication for craniectomy, type of cranioplasty material and method of construction, the time interval between craniectomy and cranioplasty, and postoperative complications.
4. Results
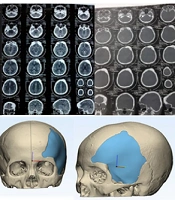
We present a report on 13 patients, who underwent cranioplasty surgery to repair extensive skull defects following meningioma surgery (Figure 1) of these patients; 5 (38%) had skull defects in the left frontotemporoparietal region, 2 (15%) had developed meningioma in the sphenoorbital region and subsequently had a skull defect in the right temporoparietal, 1 (7%) had a skull defect in the right frontal, 1 (7%) had a skull defect in the right parietal, and 4 (33%) had skull defects in the right frontotemporoparietal. In all cases, the original autologous bone flap was unavailable for reimplantation. Out of our 13 patients, 11 (84%) were female and all of them had a history of using progesterone contraception for about 10 to 30 years. The remaining two patients were male and had comorbid diabetes mellitus. The age of our patients ranged from 39 to 59 years.
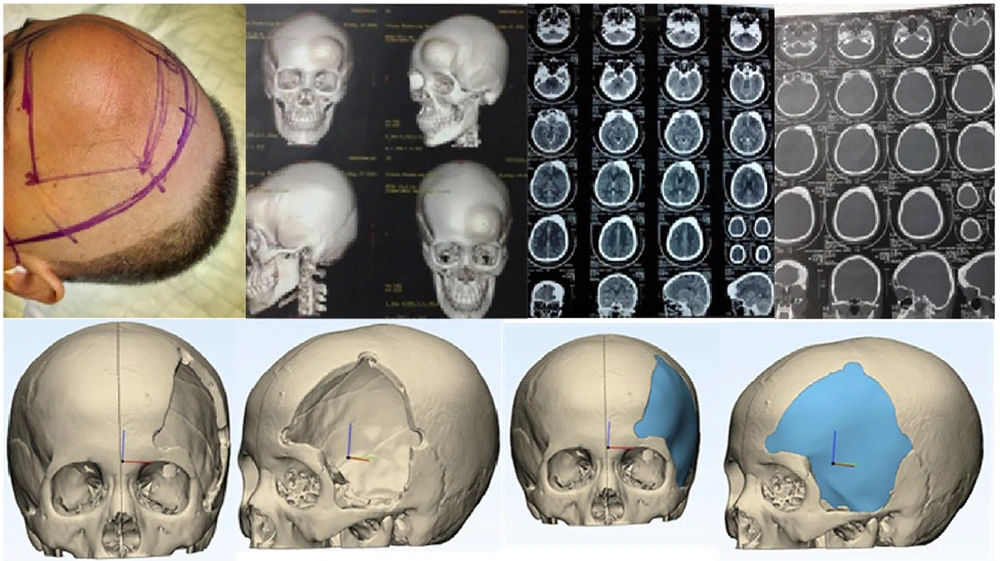
All of our patients stayed in the hospital for 3 to 5 days, after which we began outpatient care. The interval between craniectomy and cranioplasty was about one and a half months, during which we prepared the CT scan and materials for the cranioplasty. The material used was polymethyl methacrylate prostheses. Cranioplasty's preparation commences well before the actual surgery. It initiates with a pre-surgery CT scan (Figure 1) to identify the size and location of the skull defect. Subsequently, we employ CAD/CAM technology to reconstruct the defect, crafting a customized prosthesis for each patient (Figure 1).
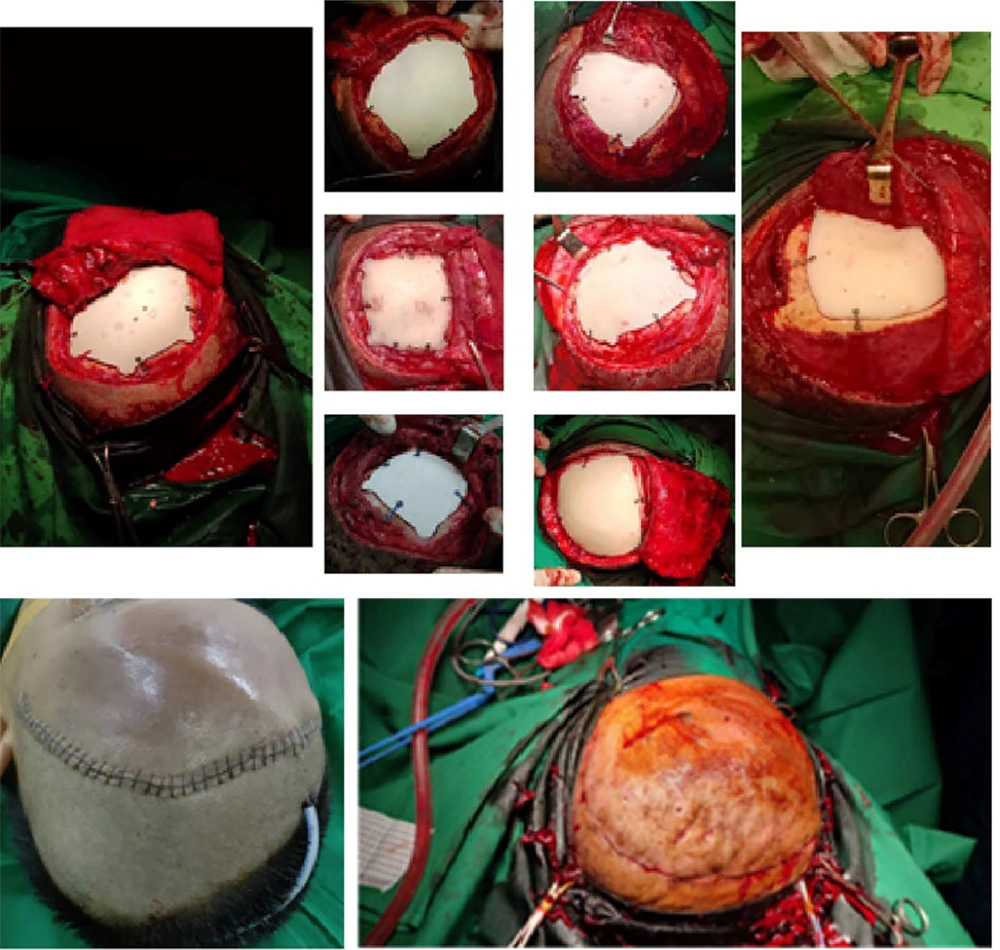
This prosthesis is, then, fabricated, using a 3D printer. On the surgery day, all patients were administered perioperative antibiotics. The prosthesis was utilized to shape a mold, which underwent sterilization and was employed during the surgery to create a PSI (Figure 2). Following the cranioplasty procedure, the patient's skull shape was restored to its original state (Figure 2).
We conducted post-operative surgery follow-up for up to 3 months. After one month of post-operative surgery follow-up, 2 (15%) out of our 13 patients developed cerebrospinal fluid leakage. The symptoms included the presence of a tumor on one side of the patient's head, which was painless, and the patient denied having any headaches, vomiting, or seizures. No neurological deficits were found during the examination, and the consistency of the tumor was soft upon palpation with no pain.
5. Discussion
The indication for craniectomy in all of our patients was meningioma. Of these patients, 84% were female and had been using progesterone contraceptives for 10 to 30 years, with an age range of 39 to 59. This finding is consistent with another study that also reported that meningioma incidence rate was higher in patients aged 40 years and older, with a rate of 18.69 per 100 000, compared to patients aged 0 to 19 years, with a rate of 0.16 per 100 000. The incidence rate of women was twice as high as that of men (10.66: 4.75 per 100 000 person-years) (8). The reason for this increased risk of meningioma in women may be attributed to the recognized influence of sex hormones on meningioma development, which has been known since the late 1920s and suggests a role in the pathogenesis of this tumor type.
A recent study indicated that more than 90% of meningiomas displayed the presence of progesterone receptors (PRs). An increased level of PRs expression has been linked with a more positive prognosis and a reduced likelihood of recurrence (8). On the other hand, the presence of estrogen receptors (ERs), found in approximately 30% of meningiomas, could potentially lead to an adverse prognosis. Numerous studies have indicated that the utilization of external hormones, like hormone therapy, could elevate the likelihood of meningioma development. The risk of meningioma occurrence in pre-menopausal women who use hormones can be as much as 2.48 times higher compared to post-menopausal women with a history of prior hormone use (9). The development of meningioma in individuals without mutations might be linked to prolonged usage of external progesterone, often found in hormonal contraceptives. This is because PRs manage transcriptional activities by interacting with ligand-dependent co-activators and/or co-repressor proteins. The research has identified 3 co-activators for PR in meningiomas, including SRC-1, AIB-1, and TIF2. Differential expression of these co-activators leads to varying PR responses. SRC-1 and TIF2 co-activators show a positive correlation with PR, while AIB-1 does not. However, AIB-1 is essential for the estrogen response pathway, which means it might be present in PR and ER-positive tumors. PRs in meningiomas are classified into PR-A and PR-B, but they differ from those found in breast tissue. PR-B is twice the size of PR-A, and while PR-A receptors are associated with Ki67, PR-B is not. Unlike in breast cancer, where PR-A reduces ER response to ligands, estrogen regulates PR-B in meningiomas. PR is more prevalent than ER in meningiomas, but its precise role in tumor development remains unclear (9).
Intrinsic factors such as neurofibromatosis 2 (NF2) gene mutations may be the primary driver in the tumorigenesis of the mutation group. Neurofibromatosis 2 is a gene situated on chromosome 22q12 that functions to suppress tumor growth. Studies in randomized meningiomas have shown that inactivation of NF2 cause of somatic mutation, epigenetic inactivation, or allelic loss of chr22q can occur in up to 60% of these cases; according to these results, it is likely that the loss of NF2 is an essential event in pathogenesis meningioma (10). Chr22q encodes the Merlin protein, a protein belonging to the BAND 4.1 FERM gene family. The Merlin protein has a fundamental role to play in connecting plasma membrane receptors with the cortical actin cytoskeleton, which indirectly connects transmembrane receptors and intracellular effectors for regulating various signaling pathways. These pathways have a crucial role in cellular processes including proliferation, survival, cytoskeletal reorganization, cell adhesion, and cell migration. The absence of NF2 can trigger the activation of multiple oncogenic pathways, including ras/mitogen-activated protein kinase, notch, phosphoinositide 3-kinase (PI3K)/AKT, hippo, and mammalian target of rapamycin (mTOR) (11).
Several epidemiological studies have furnished evidence that diabetes heightens the risk of meningioma. However, the available research does not distinctly establish a concrete link between diabetes mellitus and meningioma. A recent study has uncovered potential explanations between diabetes mellitus and carcinogenesis, including (1) the intimate correlation between diabetes and hyperinsulinemia, which influences the number of insulin receptors (insulin receptor [IR], insulin-like growth factor receptor [IGF-R]) as well as IGF was raised. In a study comparing pachymeninges to the controls, elevated levels of IGF and IGFR were observed in meningioma tissue. Insulin can stimulate IGF-R due to its structural resemblance to IR, leading to mitogenic outcomes such as cell proliferation, angiogenesis, and metastasis; (2) cancer cells require substantial glucose to sustain cell growth and mitosis via aerobic glycolysis, also called the “Warburg effect”. Hyperglycemia makes tumor growth faster through activities like proliferation, inhibiting apoptosis, and facilitating metastasis; (3) obesity has been associated with altered sex hormone metabolism, adipokine level fluctuations, insulin resistance, and chronic inflammation and has shown positive associations with meningioma risk in certain investigations (12).
In our study, we opted to use acrylic for cranioplasty due to its economic feasibility and good biocompatibility, which is consistent with recent research (13). Nonetheless, we recorded a complication rate of 15% related to acrylic cranioplasty. This outcome aligns with prior research reporting an aggregated complication rate of 22.7% (54 out of 238 cases) for PMMA cranioplasty, which falls within the documented range of 7.6% to 24%. While PMMA cranioplasty is generally regarded as a safe method, its complication rates are akin to, though not superior, those of other alloplastic techniques (14). Different investigations have demonstrated that PMMA cranioplasty tends to have fewer instances of infection-related complications compared to the utilization of titanium mesh (15).
The interval between craniectomy and cranioplasty for our patients was one and a half months, done to minimize infection and seizure risks. Research findings have indicated that infections were most commonly reported within the initial 14 days following craniectomy, while instances of hydrocephalus were prevalent within 90 days. Furthermore, the risk of seizures was observed to increase after the 90-day mark (16). The study found that functional outcomes were better for cranioplasties performed at less than 7 weeks and 7 to 12 weeks compared to those performed at over 12 weeks. However, cranioplasty should be performed immediately after brain edema has dissolved; this can make a better chance of a good neurological outcome, despite the potential for increased infection rates. Furthermore, in cases where there are underlying conditions like diabetes, thromboembolism, or colonization with multidrug-resistant pathogens, conducting cranioplasty within a timeframe of less than 7 weeks was connected to a substantial escalation in infection rates (17).
Generally, 15% of our patients developed complications, specifically cerebrospinal fluid leakage, which is consistent with a study that reported 13% of patients experiencing this complication after cranioplasty (18). Another study reported that the cerebrospinal fluid leakage rate was 7.1 % (165/2310) (19). The risk factors for developing cerebrospinal fluid leakage after craniotomy include a high BMI, smoking, dural defect, and undergoing surgery in the infratentorial region (19). There are several causes of cerebral spinal fluid leakage. Firstly, dural calcification may cause stiffness, which inhibits brain parenchyma expansion, resulting in cerebral spinal fluid leakage. Secondly, air can act as an irritant, leading to inflammation and exudate formation. Thirdly, during the process of cranioplasty, an unintended tear in the dura can lead to the accumulation of CSF or exudates produced from the damaged subgaleal area and muscles. Furthermore, synthetic implants have the potential to trigger an inflammatory response. If cerebrospinal fluid leakage is neglected, it can lead to serious complications such as neurological site infection. Several publications have identified cerebrospinal fluid leakage as the primary risk factor for neurosurgical site infections (20). Performing revision wound surgery is crucial when cerebrospinal fluid leakage is detected to prevent further complications.
Acrylic cranioplasty using CAD/CAM technology and 3D printing has shown potential for producing PSIs to repair extensive skull defects. However, careful monitoring for post-operative complications is essential. Additional research employing larger sample sizes and extended periods of follow-up is required to validate these results and assess the long-term effects of this approach.