1. Context
1.1. Natural Killer Cells: Phenotype, Biology, and Receptors
Natural killer (NK) cells, which are a critical part of the innate immune system, show intense cytotoxic responses against virus-infected cells and altered self-cells such as tumor cells without prior sensitization. For the first time, NK cells have been observed in human peripheral blood (1) and rodent splenocytes (2), whereas additional populations of NK cells have been identified to reside in multiple lymphoid and non-lymphoid tissues, including the lymph nodes (LNs), bone marrow (BM), gut, tonsils, skin, liver, and lungs (3), where they play specialized immune functions.
Natural Killer cells represent 5% to 20% of all circulating lymphocytes in humans (4), which are characterized by the expression of neural cell adhesion molecules (NCAM or CD56) and a lack of CD3 surface antigens.
Unlike T cells, NK cells lack an antigen-specific T-cell receptor (TCR); therefore, the cytotoxic or regulatory phenotypes of NK cells are determined by the expression of various activating and inhibiting receptors and interaction with their related ligands (5). The intracellular domain of activating receptors such as NKG2A, NCR (NKP44, NKP46, NKP30), immunoglobulin-like receptors (KIR), and CD16 contains an immunoreceptor tyrosine-based activation motif (ITAM) that is phosphorylated after interaction with their ligands, resulting in activating signal transduction.
Unlike T cells, NK cells lack an antigen-specific TCR; therefore, the cytotoxic or regulatory phenotypes of NK cells are determined by the expression of various activating and inhibiting receptors and their interaction with their related ligands. Activating receptors play a crucial role in controlling NK cell function. The intracellular domain of these receptors contains an ITAM that is phosphorylated after the interaction of receptor-ligand complexes and results in activating signal transduction (6). Typical activating receptors include NKG2D and natural cytotoxicity receptors (NCR: NKP44, NKP46, NKP30) KIR, and CD16.
In contrast, NK cells are negatively regulated by inhibitory receptors that present immunoreceptor tyrosine-based inhibitory motifs (ITIM) in their cytoplasmic tails. NK cells contain two types of inhibitory receptors: HLA-specific and non-HLA-specific receptors (PD-1, SIGLEC7, and IRP60).
In addition to activating KIRs, different kinds of KIR family receptors are known to induce inhibitory feedback upon interaction with class I specific HLA molecules such as HLA-A, HLA-B, and HLA-C, whereas NKG2A recognizes non-classical HLA-E molecules. According to the initial observation of Ljunggren and Karre et al., NK cells target cells lacking HLA-I expression, whereas HLA-I-sufficient cells are spared (7). Such ligands are expressed in all nucleated cells that prevent NK cell stimulation, whereas due to the missing-self recognition phenomenon, downregulation of HLA-I on tumor cells leads to increased targeting by NK cells.
1.2. Natural Killer Cell-Based Cancer Immunotherapy
Cancer is the leading cause of death and a major public health problem that is increasing all over the world (8). The most common treatments are surgery, chemotherapy, and radiation, which have difficulty completely eradicating cancer cells. Inspired by the natural capacity of our immune system to recognize and prevent tumor progression, emerging immunotherapy-based accessory immune cells such as T and NK cells are revolutionizing the clinical management of multiple tumors (8). There are several key advantages to using NK cells in immunotherapy. First, NK cells do not require a specific antigen expressed on a particular HLA allotype, making them antigens non-specific. Instead, they can recognize numerous ligands that can trigger a cytolytic response. Second, NK cells can be readily isolated and expanded outside the body, allowing their use in adoptive or autologous cell therapy. Third, NK cells have a shorter lifespan than T cells, eliminating the need for a suicide vector to prevent excessive expansion of the transferred cells (1).
Recently, T-cell transfer therapy, especially chimeric antigen receptor (CAR) T-cell therapy, as a type of immunotherapy, has become a central focus to boost the immune system’s ability to fight tumor cells. While CAR-T cell therapy has shown potential clinical benefits in cancer treatment, a major hurdle to CAR-T cell therapy, such as cytokine release syndrome (CRS), neurotoxicity, on-target/off-tumor toxicity, tumor lysis syndrome (TLS), and anaphylaxis has also been reported. As an alternative to CAR-T cell therapy, CAR-NK cells not only overcome these limitations but also present additional major advantages (9).
1.3. Chimeric Antigen Receptor
Chimeric Antigen Receptor (CARs) are genetically engineered receptors that re-direct NK cells with a high ability to target certain proteins in cancer cells. For example, Her2 and CD19 are the most expressed targets for breast and B cell malignancies and are widely targeted by CAR-NK cells.
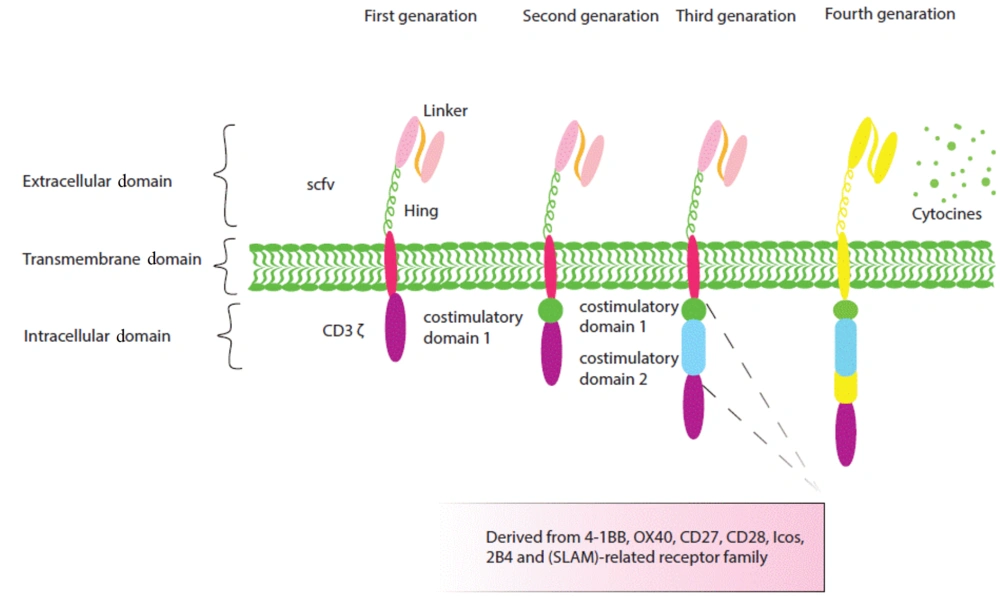
As represented in Figure 1, functional CAR molecules consist of 3 regions: Extracellular, transmembrane, and intracellular domains. The extracellular antigen-binding domain is made up of the single chain fragment variant (SCFV) with a linker between the heavy and light chains, as well as the hinge region such as CD8, CD28, DAP12, IgG1, and IgG4 that causes CAR stability and flexibility to easily access the target. Extracellular and intracellular regions join each other by a transmembrane zone that transduces activation signals to intracellular activation signals.
Structure of the first, second, and fourth generations of chimeric antigen receptors (CARs). Chimeric antigen receptors are composed of an extracellular antigen-binding domain, a transmembrane domain, and an intracellular domain. The extracellular region contains a chain fragment of the antibody variable region (ScFv) or a functional domain of a specific ligand. The intracellular region consists of a signaling domain (first generation) and a stimulatory domain (second generation) or two (third generation). Fourth-generation CARs are designed to express molecules such as cytokines.
As represented in Figure 1, functional CAR molecules consist of 3 regions: Extracellular, transmembrane, and intracellular domains. The extracellular antigen-binding domain is made up of the single chain fragment variant (SCFV) with a linker between the heavy and light chains, as well as the hinge region such as CD8, CD28, DAP12, IgG1, and IgG4 that causes CAR stability and flexibility to easily access the target. Extracellular and intracellular regions join each other by a transmembrane zone that transduces activation signals to intracellular activation signals.
The intracellular region, which mainly mediates CAR-NK cell cytotoxicity, includes activation signaling domains, and CAR generation is determined by the number of these domains (10). CARs are identified by their different generation. The first generation of CARs has only one activating signal; for example, the first CAR-NK cell generation that overcomes leukemia resistance consists of only a CD3 zeta(ζ) signaling moiety (11).
1.4. Different Sources of NK Cells for CAR-NK
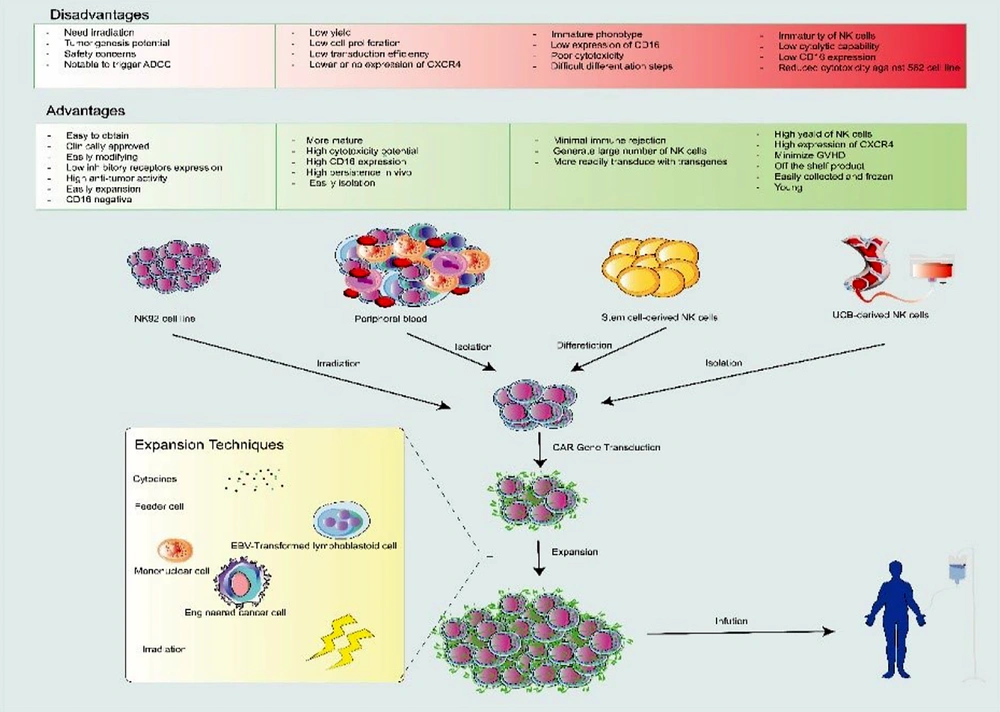
As displayed in Figure 2, allogeneic NK cells for immunotherapy can be derived from different sources, such as NK cell lines, induced pluripotent stem cells (iPSCs), cord blood (CB), hematopoietic stem cells (HSCs), and peripheral blood (PB). Obtaining large numbers of NK cells and being time-consuming are the common limitations of NK cell sources while cell lines overcome these problems (12).
Sources and generation procedure of CAR-NK cells. Isolated or established natural killer (NK) cells from various sources (e.g., PB, UCB, NK cell line, and Stem cell-derived NK cells) can be activated and genetically modified with CAR-expressing vectors (e.g., lentivirus or retrovirus) and, then, expanded by some techniques (e.g., using cytokines, irradiation, feeder cells such as mononuclear cell, EBV transformed lymphoblastoid cells or engineered cancer cell). UCB, umbilical cord blood; PB, peripheral blood; CAR-NK, chimeric antigen receptor-natural killer cells.
Many NK cells, including KHYG-1, NK92, NKL, NKG, and YT cells with notable advancement have been studied.
1.5. NK92
The NK92 cell line not only shows high anti-tumor activity by direct cytotoxicity but also could be an ideal CAR carrier because of its easy modification. The first CAR-NK92 cells targeting HER2 were reported by Uherek et al. Other studies also confirmed the efficacy and safety of CAR-based NK92 cells in preclinical and clinical trials by targeting hematological malignancies such as acute myeloid leukemia (CD33), lymphoma (CD19), myeloma (CSI), and other solid tumors such as glioblastoma (EGFR), ovarian cancer (mesothelin), and prostate cancer (EPCAM) (13).
1.6. Peripheral Blood
The most available source of NK cells is peripheral blood, which can be easily isolated from healthy donors (allogeneic PB-NK) or from the patient itself (autologous PB-NK). Using autologous NK cells is typically not impressive, as they are functionally inhibited after encountering self-MHC antigens; therefore, for immunotherapy purposes, allogenic PB-NK cells could be more effective (14). Furthermore, because of the low number of NK cells after isolation, they are routinely expanded by various expansion protocols, which is another difficulty because prolonged culture causes telomerase shortening and subsequently reduced cytotoxicity (15).
1.7. Umbilical Cord Blood-Derived NK Cells (UCB-Derived NK Cells)
UBC-derived NK cells as a potential "off the shelf" product have recently been noticed (16). The NK cells in UBC can be easily collected and frozen with high proliferation potential and number compared to PB-isolated NK cells. For instance, only 10% of one cord blood unit is sufficient to generate more than 109 NK cells after 2 weeks (17). In addition, according to a study, more than 100 doses of NK cells were collected just from a single UBC unit for clinical usage. Although a minority of the preclinical studies applied UBC as an NK cell source, the safety and potency of CB-NK cells were demonstrated in UBC-NK trials (12). A recent phase I/II study showed that CAR-NK targeting CD19-expressing malignancies can persist for more than 270 days in vivo. This group also demonstrated that most 11 patients with relapsed/refractory CD19-positive cancer reacted CAR-NK without any toxic effects (18).
1.8. Stem Cell-Derived NK cells
The other potential source for CAR-NK generation is pluripotent stem cells, including induced pluripotent stem cells (iPSCs) and human embryonic stem cells (hESCs), which have emerged as favorable sources for the generation of off-the-shelf and engineered cell products with high antitumor function (19). For CAR-NK cell therapy, the major sources of donor NK are peripheral blood (PB) and umbilical cord blood (UCB), which have their challenges. Although in vitro expansion from a single donor may be adequate for a limited number of patients, it is not enough for an off-the-shelf allogenic commercial product (20).
Despite these advantages, there are still some challenges ahead in iPSC-CAR-NK cells in clinical applications, including the stop of CAR-iPSC-NK cell proliferation in vivo after exogenous administration of cytokines and the immunogenic and malignant transformation potential of these cells (12).
1.9. CAR Transduction Platform in NK cells
Efficient and secure genetic delivery systems are crucial for the successful production of genetically modified cells; therefore, different approaches, including viral and nonviral platforms, are applied for CAR delivery (21). Notably, the considerable difference between these technologies is the duration of CAR expression stability.
1.10. Retroviruses
For decades, various studies have shown highly efficient CAR transduction into pre-stimulated NK cells by using a single dose of retroviral vectors ranging from 27 % to 52% (22). However, Imamura et al. achieved more impressive transduction (approximately 70%) of IL15 expression using a retroviral vector-based method. In addition, long-term persistence of retrovirally CAR-engineered NK cells has been reported. According to a recent clinical trial targeting lymphoid tumors (non-Hodgkins lymphoma and CLL), 7 of 11 patients completely recovered after infusion of retrovirus-transduced anti-CD19 CAR cord blood NK cells, and remarkably after a 1-year follow-up, expanded CAR-NK cells were detectable in the peripheral blood of treated patients (18).
1.11. Lentivirus
As with retroviruses, lentiviruses have also been greatly exploited for decades, with the difference that lentiviruses are not dependent on cell cycle progression and can transduce cycling and ever-non-cycling cells with higher transduction efficacy. Lentiviruses also possess certain other benefits such as low immunogenicity and the ability to integrate transgenes into the host genome, which can result in the permanent expression of the transgene (23).
1.12. Non-viral Approaches
NK cells have demonstrated a higher level of resistance to viral infection than T cells. This resistance may be attributed to the inherent ability of NK cells to protect against viral infections (24). In addition, alternative methods such as transposon vectors and electroporation of DNA or RNA plasmids have gained interest due to their safety, outstanding design flexibility, ease of producing therapeutic cells on a large scale at a reasonable speed, and low cost (25).
1.13. Non-viral Electroporation (m-RNA Electroporation)
Electroporation, a commonly used transient technique, involves the use of electric pulses to make the cell membrane permeable and introduce genetic material into the cells. Various studies have relied on electroporation (26). Electroporated cargo mostly includes CAR-encoding mRNA or plasmids. Despite the satisfactory effectiveness of electrotransfection in delivering CAR-encoded DNA, various studies have indicated a potential danger of growth inhibition and mortality in NK cells, which hampers the application of DNA. In contrast, when DNA-electroporated NK cells were compared with mRNA-electroporated NK92 cells after 24 h, it was found that the DNA-electroporated NK92 cells showed 2- or 3-times lower cell viability (27). Therefore, more focus has been placed on mRNA-based electroporation because of its superior transfection efficiency (up to 95%) (28).
1.14. Nanoparticles
Viral transduction and electroporation techniques have been successful in achieving high levels of gene expression in NK cells. However, these methods are time-consuming and require specialized equipment and laboratory setups. In contrast, mRNA-carrying nanoparticles, which can be easily synthesized in the lab, have shown promise in CAR cell technology (29). These nanoparticles can be made from various materials such as polymers or ionizable lipids. Kim et al. used polymeric nanoparticles complexed with PDNA EGFR CAR to efficiently transfect NK92MI cells and demonstrated anti-oncogenic activity in a breast cancer model (30). Ionizable lipid nanoparticle platforms are particularly attractive because of their ability to provide stable formulation, low toxicity rates, and effective endogenous cellular internalization (31).
1.15. Transposons
The use of transposons has gained significant attention over traditional methods because of several advancements. These include their high effectiveness, ability to transduce CAR transgenes at specific locations (32), transfection of more than 10 kb gene fragments, lower immunogenicity, and cost-effectiveness (33).
Transposons consist of mobile plasmids with 2 terminal inverted repeats (TIRL) that carry the enzymatic gene and the desired sequence. The transposase enzyme facilitates the cutting and pasting of these desired elements into the host genome (34). Two commonly used transposons are Sleeping Beauty (SB) and PiggyBac (PB), which have shown promise in therapeutic cell manufacturing. SB-based CAR-modified cells are currently being clinically tested because of their potential and safer integration profile. However, generating CAR-NK cells using transposons is more challenging than generating primary NK cells. Nonetheless, there have been studies using transposon systems to generate CAR-NK cells (35).
1.16. Crisper-cas9
The clustered regulatory interspaced short palindromic repeats (CRISPR) associated protein (CAS9) system has greatly advanced biomedical research by providing a powerful tool for genome editing (36). This technology involves introducing cas9 as a nuclease along with guide RNA (sgRNA) to a specific location in the genome, causing double-strand breaks. The desired gene can, then, be integrated through endogenous DNA repair mechanisms, such as homologous recombination (HR) or non-homologous recombination (NHEJ). Initially, this technology was used to knock out different genes; for example, as CD38 is expressed both on NK cells and AML cells, in one study to disrupt CD38 of NK cells. These cells were electroporated with sg RNA-cas9 to prevent fratricide of NK cells when used along with the anti-CD38 antibody daratumumab (36). Another study successfully knocked out approximately 80% of the NKG2A-encoding killer cell lectin-like receptor C1 (KLRC1) locus in primary NK cells using CRISPR/CAS9 (37). In recent times, the technique of CRISPR/CAS9 has also been used to incorporate CRISPR/CAS9 plasmid, which has caused NK cells to be resistant to this technology, which is a major issue for gen-editing-based immunotherapy using CRISPR/CAS9. Thus far, numerous approaches have been used in an attempt to create a secure and effective delivery system (38). However, when the expression levels of the CAR gene can vary when it is delivered through transposon-based random insertion, electroporation of cas9 single guide RNA complexes has been established as a method to deliver large-sized CRISPR/CAS9 plasmids into NK cells (39).
1.17. Trogocytosis
Trogocytosis refers to the exchange of membrane patches between immune cells and target cells. When an NK cell interacts with a target cell, a strong immune synapse is formed, enabling the transfer of small membrane patches between two cells. As a result, surface molecules from the target cell can be detected on the surface of NK cells. This process has been used to modify the expression of certain molecules on NK cells, enhancing their ability to destroy target cells (40).
Transfer of the chemokine receptor CCR7 from engineered K562 cells to human NK cells through trogocytosis was intentionally facilitated. This process, as observed in a study conducted by Somanchi et al., led to an increase in CCR7 expression in 80% of NK cells after being co-cultured for 1 h. Additionally, this resulted in improved migration to lymph nodes (41).
1.18. Microfluidic-Based Cell Squeezing
Devices that use microfluidic technologies to squeeze cells have been quickly developed to effectively deliver large and small molecules into different types of cells. This method works by mechanically modifying the cells, temporarily making their cellular membranes permeable. Unlike electroporation, this technique has a minimal negative effect on the functionality of immune cells (42). In the future, this approach is anticipated to be a valuable strategy for delivering substances without viruses. However, more research is needed to understand how this technique specifically affects immune cells such as NK cells (43).
1.19. CAR-NK vs. CAR-T and CAR-M for Cancer Immunotherapy
In recent years, considerable progress has been made in CAR-T cell immunotherapy, particularly for the treatment of hematological malignancies. To date, 6 CAR-T cell therapies, namely Kymriah, Yescarta, Breyanzi, Tecartus, Carvykti, and Abecma have been approved by the FDA. Despite impressive results, there are unwanted disadvantages and obstacles associated with CAR-T therapy (44). The key challenges faced in clinical applications are as follows: First, its manufacturing processes are intricate and centralized and, thus, require a high cost, which reduces patients’ access to treatment. The next issue is to reduce autologous T-cell efficacy in heavily treated patients (45). Although allogeneic CAR-T cells can solve this challenge, GvHD and host allograft rejection are the two main limitations. Unlike the successful effect of CAR-T in hematological cancers, it has minimal function in solid cancers (46). Common CAR‐T side effects are cytokine release syndrome (CRS) and neurotoxicity (47). Target antigen loss provides a great opportunity for tumor immune escape, which occurs after CAR T-cell therapy. The exhaustion phenotype and limited durability of CAR-T cells suppress their function. Because of these deficiencies, the clinical use of CAR-T is limited. Hence, to solve these challenges, the use of alternative cell sources such as NK cells and macrophages is an ideal and promising option because of their desirable properties. Therefore, we compared the potential benefits of CAR-NK and CAR-M cells with those of CAR-T cells.
1.20. CAR-NK Cell Therapy: Advantages and Limitations
The great achievements in CAR-T cell therapies have led to the development of subsequent generations of NK-based cell therapies (48). Given the above limitations, CAR-NK cells may be superior to CAR-T cells, especially in cancer immunotherapy, as shown in several clinical trials. CAR structures for NK cells can be designed similar to CAR in CAR-T cells, and T cell technology is easily applied to NK cells.
1.21. Advantages
Evidence-based CAR-NK cell therapy is an innovative strategy with great potential for cancer immunotherapy (49). CD8+ T cells and NK cells use the same mechanisms to kill infected and cancer cells, but they have drastically different mechanisms of target recognition (50). Both kill target cells through lysosomal granule release and the Fas ligand and produce cytokines. The significant advantages offered by CAR-NK cells over CAR-T cells make them a novel candidate in the context of cancer immuno-cell therapy (51). First, they can quickly identify and kill their aims without prior sensitization. Although they do not require HLA matching, they cannot cause GvHD; therefore, they might be generated on an off-the-shelf platform for wider clinical use. As a result, NK cells can be generated from existing cell lines as well as from allogeneic NK cells that have mismatched major histocompatibility complex (MHC) molecules (52). As a result, there are broad cell sources used to construct CAR-NK, but CAR-T cells cannot be used for allogeneic T therapy because of GVHD (52). Second, triggered NK cells can destroy targeted cells not only through the CAR pathway but also through other pathways, such as the release of proinflammatory cytokines (perforin and granzymes) and another mechanism using antibody-dependent cellular cytotoxicity (ADCC) through Fas ligand (FasL) expression and TNF-related apoptosis-inducing ligand (TRAIL). Third, the main mechanisms of tumor escape from CAR-T cells occur through decreased MHC-I, whereas CAR-NK cells maintain their innate cytolytic capacity via self-missing recognition (53). Fourth, studies have shown that, unlike the activation of CAR-T cells that release proinflammatory cytokines (including IL-1, IL-2, IL-6, and IL-15), activated NK cells produce different cytokines, such as IFN-γ, TNF-α, interleukin-3, and GM-CSF; therefore, CRS and neurotoxicity rarely occur during CAR-NK cell therapy.
1.22. Limitations
Most obstacles associated with CAR-T cell therapy are exerted by CAR-NK cells, such as target antigen selection, CAR design, diversity of antigens, post-infusion challenge generation, such as NK cells moving to tumor locations, and a belligerent tumor microenvironment (52). First, as discussed above, CAR structures in NK cells are similar to those of CAR-T cells, although they are low-quality selections for use in NK cells (54). Second, transduction methods, including viral transfection and electroporation, have low efficiency (55). Third, a lack of cytokine support leads to limited durability in vivo. Although desirable, this can reduce the influence of CAR-NK cell immunotherapy (21). Fourth, in ex vivo-expanded human NK cells, unlike T cells, the number of NK cells in the blood is limited; therefore, to reach a large scale, the use of expansion techniques is required. Fifth, the tumor microenvironment is a major problem in reducing the ability of CAR-NK cell therapy to eliminate target cells, including immunosuppressive cell types.
1.23. Pre-clinical Studies
Preclinical studies have demonstrated very promising anti-tumor results for CAR-NK cells in hematological and solid tumor cells. Among multiple sources of NK cells for CAR modification, the NK-92 cell line is largely used in both hematological and solid tumors. Some preclinical studies have investigated the efficacy and safety of CAR-NK cells against tumor antigens such as HER2, CD20, CD19, and CD244 (56).
1.24. Preclinical Studies of Hematologic Cancers
The majority of preclinical and clinical success on CAR-NK cells has been performed on hematologic malignancies (57). NK cell CAR-specific targets, including CD19, CD138, CD20, and Flt3, have been successfully used to treat hematologic malignancies (58). To direct cytotoxicity against B-cell malignancies, NK cells have been genetically manufactured to represent CARs. CD19-CAR-NK cells can be generated from iPSCs, PB, and UCB, which have been shown to eliminate B-cell malignancies in vitro (59). The most common purpose of hematological malignancies is the CD19 antigen (B cell malignancies). NK‐92 cells have shown effective cytotoxic activity against several lymphoblastic cells expressing CD19 (11). Dual‐targeting CAR approaches targeting CD19 and CD138 antigens used to produce CAR-NK-92 cells showed in vitro cytotoxicity and purposed tumor killing (60).
1.25. Preclinical Studies of Solid Cancers
Although CAR-T-cell immunotherapy has revealed considerable progress in treating hematologic malignancies, solid tumor limitations such as immunosuppressive TME and poor perfusion to the tumor have not been solved (61). In preclinical studies, CAR-NK cells can use promising immunotherapy strategies, such as overexpression of the chemokine receptors CXCR4 or CXCR1, which provide particular chemotaxis to remedy these limitations (62). Thus, multiple solid tumors such as glioblastoma, breast cancer, colon cancer, hepatocellular carcinoma, pancreatic cancer, and head and neck squamous cell carcinoma (HNSCC) are the researched options for CAR-NK-cell preclinical study (63). All preclinical studies are summarized in Table 1.
| Malignancy | Target | Source of NK Cells | Result | Reference |
|---|---|---|---|---|
| B-cell malignancies | CD19 CD20 Flt3 | NK-92, PB-NK or CB-NK NK-92 NK-92 | NK92-CD19-CD3ζ cells have cytotoxic effects against B-lineage malignancy. Transfection of CD19-41BB-mRNA into NK cells with electroporation technology illustrated that 61.3% of cells stably present anti-CD19 CAR, following that, optical in vivo imaging proved that CAR-NK cell therapy is effective in reducing the growth rate of leukemia xenografts. In vitro, NK-92 cells carrying anti-CD19 CAR demonstrated excellent cytolytic activity against previously resistant CD19-positive B-ALL and primary chronic lymphocytic leukemia (CLL) cell lines. CAR-NK-92 cells with CD20 and Flt3 expression in immunotherapy against B-cell tumors that lead to degranulation of NK cells and selective cytotoxicity. In vitro, anti-CD20 CAR-adjusted and expanded peripheral blood NK cells reduce the tumor burden of CD20+ B-NHL cells in vitro. Anti-CD20 CAR-NK cells have higher anti-tumor toxicity than anti-CD20 antibodies in chronic lymphocytic leukemia (CLL) cells. | (61, 64) |
| T-cell malignancies | CD3 CD5 CD7 | NK-92 NK-92 NK-92 | CAR-adjusted NK cells or NK-92 cell lines to target CD3, CD5, and CD7 revealed important anti-tumor activity against T-cell malignancies. | (60, 65) |
| Multiple myeloma | CD138 CS1 BCMA NKG2D | NK-92 NK-92 NK-92 PB-NK | CS1-CAR NK-92 cells enhanced IFN-γ production and cytotoxic activity in response to primary multiple myeloma (MM) tumor cells with high expression CS1. CD138-CAR-NK-92 eliminates myeloma cells and prolongs the survival rate of MM mice. The therapy with BCMA-CAR-NK cells with co-expressing CXCR4 reduced the in vivo progression of MM and the mice’s survival was extended. | (66, 67) |
| AML | NKG2D | PB-NK | NK cells derived from peripheral blood with NKG2D-specific CAR exhibited their ability to lyse AML tumor cells in vitro and in a xenograft model. | (68) |
| Glioblastoma | HER2 EGFR and/or EGFRvIII | NK-92 NK-92, NKL, KHYG1 or YTS | Intravenous injection of EGFRvIII-directed CAR-NK cells with overexpressing of CXCR4 receptor, mice was treated and prolonged their survival. Bispecific CAR-NK-92 cells by EGFR and EGFRvIII-driven were injected intracranially in xenograft mouse glioma models, prolonged the mouse survival and enhanced cytotoxicity and IFN-g secretion. Also, ErbB2(HER2)-CAR-NK-92/5.28. z cells with CD3ζ and CD28ζ signaling domains have shown that they can eradicate ErbB2-positive glioblastoma cells in vitro. | (69, 70) |
| Ovarian cancer | HLA-G CD24 αFR CD133 CD44 Mesothelin | PB-NK NK-92 NK-92 NK-92 NK-92 iPSC-NK or NK-92 | αFR (folate receptor)-targeted CAR-NK-92 cells showed cytotoxicity specific to antigen and significantly increased survival in animal models.MSLN-CAR NK cells derived from both iPSC and NK-92 cell lines illustrated the effective elimination of MSLN-positive OC cells (OVCAR-3 and SK-OV-3). | (32, 71, 72) |
| Breast cancer | EpCAM B7-H6 TF HER2 EGFR and/or EGFRvIII | NK-92 NK-92 NK-92 NK-92 NK-92 or PB-NK | NK-92/31.28. z cells were applied against EpCAM and demonstrated potent cytotoxicity and cytokine secretion. B7-H6 and TF as recent targets in CAR-NK immunotherapy enhanced tumor-killing capacity of specific CAR-NK-92 cells. The NK-92-scFv (FRP5)-zeta cell line presenting a chimaera HER2 antigen receptor in breast cancer with HER-2 mutation resulting in vivo antitumor activity. EGFR-CAR-NK cells could lyse TNBC in vitro and curtailing tumor growth. Dual-specific CAR-NK-92 cell, capable of recognizing both EGFR and EGFRvIII, has shown high cytotoxic and produce IFN-γ to battle breast cancer cells. | (73, 74) |
1.26. Clinical Studies
Confirmation of the safety and effectiveness of non-engineered NK cells and reassuring evidence from preclinical investigations of CAR-NK treatments support the clinical benefits of CAR-NK cells in patients. Since the initial recorded CAR-NK cell trial in 2009 (NCT00995137), there have been 60 registered trials on clinicaltrials.gov. Most of the clinical studies are related to recent years (mainly between the years of 2021-2023), as 15, 14, and 9 clinical trials have been registered on clinicaltrials.gov in 2021, 2022, and 2023, respectively. Among the registered studies, 10 trials had an unknown status, and 2 trials were withdrawn. Although 4 studies have completed status on clinicaltrials.gov, there is a lack of accessible information regarding these trials. The majority of related clinical trials focus on hematologic cancers, particularly B-cell lineage leukemia/lymphoma. Despite CD19 being the most common targeted antigen, CD20, CD22, CD2, NKG2D, BCMA, CD5, CD70, CD33, and CD123 are other markers used for targeting lymphoproliferative tumors.
In this regard, during phase 1 and 2 clinical trials, CB-derived CD19-CAR-NK using K562/mbIL-21/41BBL was administered to 11 volunteers with r/r CD19-positive B-cell malignancies. Following the lymphadenopathy regimen, the injection of engineered cells was performed at only 1 of 3 dosages (10×104, 10×105, or 10×106 CAR-NK cells/kg) of a single infusion. The findings demonstrated that 73% of the patients had objective responses with no serious side effects. In addition, at all applied doses, responses were quick and visible after 30 days of infusion (18). Despite the favorable results of this trial, Karadimitris in 2020 presented a high percentage of transient clinical responses to CB-derived CAR-NK cell-based immunotherapy (75). In addition, preliminary findings from a phase I clinical study [NCT04245722] that evaluated the effect of iPSC-derived CD19-directed NK cells (FT596) on 20 patients with r/r B-cell lymphomas (BCLs) and CLL have been published. To increase the efficacy of NKs, the cells were modified for 3 antigens: CD19, CD16, and IL-15 receptors and used in combination with monoclonal antibodies. Data, while exhibiting (no evidence of immune effector cell–associated neurotoxicity syndrome, GVHD, and cytokine release syndrome), displayed an objective response in 11 patients from all 17 evaluable subjects (76). Another equivalent study used a single dose of FT596 (30×106 cells) in a female patient aged 76 years with diffuse large B-cell lymphoma. The results illustrated the early clinical benefits of FT596, such as safety, no GVHD, no neurologic toxicity, no dose-limiting toxicities, and no remarkable adverse reactions related to cell therapy. According to the 2014 Lugano criteria, the measurement of the tumoral response at the one-month mark revealed a partial response, with a decrease of more than 70% in 18F-Glu absorption and a reduction of more than 50% in tumoral size. Furthermore, a phase I trial of this protocol [NCT04245722] is in progress, with 285 individuals expected to participate (77).
Notably, 17 clinical trials are related to solid tumors, including brain, lung, prostate, colorectal, ovarian, prostate, pancreatic, and other types. They mostly selected HER2, NKG2D, mesothelin, CCCR, Claudin6, ROBO, DLL3, PSMA, and CD70 markers to target tumor cells using CAR constructs. One promising target for CAR-NK treatment of solid malignancies is the Robo-1 antigen. A case report study intravenously infused 1×109 Robo1- specific CAR-NK cells into a 46-year-old man with pancreatic ductal adenocarcinoma followed up every two weeks. The evaluation showed 5 months of controlled pancreatic lesions, no significant adverse outcomes, and an overall survival time of 8 months. Based on the noted research and preclinical experiments, 3 phase I/II clinical studies [NCT03941457, NCT03940820, and NCT03931720] in China are recruiting for the investigation of ROBO-1-specific CAR-NK cells in solid tumors (57). In a similar strategy, PD-L1 antigen was considered for immunotherapy of solid cancers, so PD-L1 targeting CAR-NK combined with IL-15 superagonist (N-803) and pembrolizumab is presently currently appraised for gastroesophageal junction and HNSCC tumor participants in the form of a phase II clinical trial (63). Another trial [NCT03415100] was conducted to assess the safety and feasibility of local administration of allogeneic or autologous NKG2DL-engineered CAR-NK cells in 3 subjects with metastatic colon cancer. Two patients achieved a decrease in the production of ascites and a remarkable drop in the number of tumor cells in ascites specimens; however, the third patient, who had liver metastases, indicated a complete metabolic response and rapid tumor regression of liver lesions (78). Furthermore, the FDA approved the FT536 product (by Fate Therapeutics) for patients with advanced solid malignancies in January 2022 (NCT05395052). This product is an allogeneic iPSC-derived CAR-NK cell line targeting the alpha-3 domain of MHC class I chain-related proteins A (MICA) and B (MICB). A comprehensive overview of all clinical trials is presented in Table 2.
| Clinical Trial Identifier | Status | Clinical Trial Phase | Disease | Antigen | NK Source | Number Enrolled | Interventions | Dosage | Starting Date | Location |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT00995137 | Completed | Phase I | B-Lineage ALL | CD19 | Unknown | 14 | Anti-CD19 CAR-NK cells | Unknown | October 2009 | United States |
| NCT01974479 | Suspended for an interim review of (CAR) CD19 research strategy | Phase I | B-Lineage ALL | CD19 | Allogeneic | 20 | Anti-CD19 redirected NK cells | 0.5 -5×107/kg, and up to 1×108/kg | September 1, 2013 | Singapore |
| NCT02839954 | Unknown | Phase I/II | MUC1 positive r/r solid tumor | MUC1 | Unknown | 10 | Anti-MUC1 CAR-pNK cells | Unknown | July 21, 2016 | China |
| NCT02944162 | Unknown | Phase I/II | AML | CD33 | NK-92 | 10 | Anti-CD33 CAR-NK cells | Unknown | October 2016 | China |
| NCT03056339 | Completed | Phase I/II | r/r B Lymphoid Malignancies | CD19 | CB | 44 | iC9/CAR.19/IL15-Transduced CB-NK Cells | 1×106 | June 21, 2017 | United States |
| NCT03383978 | Recruiting | Phase I | Glioblastoma | HER2 | NK-92 | 42 | NK-92/5.28.z | 1×107; 1×108 | December 1, 2017 | Germany |
| NCT03415100 | Unknown | Phase I | Metastatic Solid Tumors | NKG2D-L | Autologous or allogeneic | 30 | CAR-NK cells targeting NKG2D ligands | Unknown | January 2, 2018 | China |
| NCT03656705 | Enrolling by invitation | Phase I | NSCLC | |||||||
| NK-92 | 5 | CCCR-NK92 cells | 1×107; 1×108 | September 29, 2018 | China | |||||
| NCT03692663 | Recruiting | Early Phase 1 | mCRPC | PSMA | Unknown | 9 | TABP EIC | 0.5, 10, and 30 × 106 | December 1, 2018 | China |
| NCT03824964 | Unknown | Early Phase 1 | r/r B cell lymphoma | CD19/CD22 | Unknown | 10 | Anti-CD19/CD22 CAR-NK Cells | 50 – 600 × 103/kg | February 1, 2019 | unknown |
| NCT03692637 | Unknown | Early Phase 1 | Epithelial ovarian cancer | Mesothelin | Autologous | 30 | anti-Mesothelin CAR-NK Cells | 0.5 – 3 × 106/kg | March 2019 | unknown |
| NCT03692767 | Unknown | Early Phase 1 | r/r B cell lymphoma | CD22 | Unknown | 9 | Anti-CD22 CAR-NK Cells | 50 - 600 × 103/kg | March 2019 | unknown |
| NCT03690310 | Unknown | Early Phase 1 | r/r B-Cell Lymphoma | CD19 | Unknown | 9 | Anti-CD19 CAR-NK Cells | 50 - 600 × 103/kg | March 2019 | unknown |
| NCT03940833 | Unknown | Phase I/II | MM | BCMA | NK-92 | 20 | BCMA CAR-NK 92 cells | Unknown | May 2019 | China |
| NCT03931720 | Unknown | Phase I/II | Malignant Tumor | ROBO1 | Unknown | 20 | BiCAR-NK/T cells (ROBO1 CAR-NK/T cells) | Unknown | May 2019 | China |
| NCT03940820 | Unknown | Phase I/II | solid tumors | ROBO1 | Unknown | 20 | ROBO1 CAR-NK cells | Unknown | May 2019 | China |
| NCT03941457 | Unknown | Phase I/II | Pancreatic Cancer | ROBO1 | Unknown | 9 | BiCAR-NK cells (ROBO1 CAR-NK cells) | Unknown | May 2019 | China |
| NCT03579927 | Withdrawn (lack of Funding) | Phase I/II | B-Cell Lymphoma | CD19 CD28-zeta-2A | CB | 0 | CD19-CD28-zeta-2A-iCasp9-IL15-transduced CB-CAR-NK cells | Unknown | October 3, 2019 | United States |
| NCT04639739 | Not yet recruiting | Early Phase 1 | NHL | CD19 | Unknown | 9 | Anti-CD19 CAR-NK | 2 × 106/kg, 6 × 106/kg, 2 × 107/kg | December 17, 2020 | China |
| NCT05215015 | Recruiting | Early Phase 1 | AML | CD33 and CLL1 | Unknown | 18 | Anti-CD33/CLL1 CAR-NK Cells | 2 × 109; 3×109; 18×108 | November 30, 2020 | China |
| NCT04623944 | Recruiting | Phase 1 | Hematological Malignancies or Dysplasia | NKG2D | Allogeneic PBMC | 90 | CAR-NK cells targeting NKG2D ligands | 1×108 NK cells (2 ×106/kg for patients < 50 kg), 1.5×108 NK cells (3 ×106/kg for patients < 50 kg) | September 21, 2020 | United States |
| NCT04245722 | Active, not recruiting | Phase 1 | B-cell Lymphoma and CLL | CD19 | iPSC | 98 | FT596 + Cyclophosphamide + Fludarabine + Rituximab + Obinutuzumab + Bendamustine | Unknown | March 19, 2020 | United States |
| NCT04747093 | Unknown | Phase I/II | B cell malignancies | CD19 | Induced-T Cell Like NK | 12 | Induced-T Cell Like NK (CAR-ITNK cells) | Unknown | January 29, 2021 | China |
| NCT04796675 | Recruiting | Phase 1 | hematological malignancies. | CD19 | CB | 27 | CAR-NK-CD19 Cells | 0.01 × 107, 0.1 × 107, 1 × 107 | April 10, 2021 | China |
| NCT04887012 | Recruiting | Phase 1 | B-NHL | CD19 | Unknown | 25 | Anti-CD19 CAR-NK | Unknown | May 1, 2021 | China |
| NCT05020678 | Recruiting | Phase 1 | B-cell Malignancies | CD19 | Allogenic PBMC | 150 | Anti-CD19 CAR-NK Cells | 3 × 108, 6 × 106/kg for patients < 50 kg | August 20, 2021 | United States |
| NCT05248048 | Recruiting | Early Phase 1 | metastatic colorectal cancer | NKG2D | Unknown | 9 | NKG2D CAR-NK Cell | Unknown | September 13, 2021 | China |
| NCT05008536 | Recruiting | Early Phase 1 | MM | BCMA | CB | 27 | Anti-BCMA CAR-NK Cells | 1 – 3 × 106/kg, 3 - 6×106/kg, 0.6 - 1.2×107/kg | October 1, 2021 | China |
| NCT05247957 | Terminated | Phase I | AML | NKG2D | CB | 9 | NKG2D CAR-NK | 2 × 106/kg, 6 × 106/kg, 18 × 106/kg | October 13, 2021 | China |
| NCT05 379647 | Recruiting | Phase 1 | B-Cell Malignancies | CD19 | allogeneic | 24 | QN-019a (allogeneic CAR-NK cells targeting CD19)+ Anti-CD20 Monoclonal Antibodies | Unknown | November 4, 2021 | China |
| NCT05020015 | Recruiting | Phase 2 | r/r B-cell NHL | CD19 | CB | 242 | TAK-007 | 200 × 106, 800 × 106 | November 22, 2021 | Japan |
| NCT05137275 | Recruiting | Early Phase 1 | Locally Advanced or Metastatic Solid Tumors | 5T4 | Allogeneic | 56 | Anti-5T4 CAR-raNK Cells | 3.0 × 109, 6.0 × 109, 9.0 × 109 | November 24, 2021 | China |
| NCT05182073 | Recruiting | Phase 1 | r/r Multiple Myeloma | BCMACD38 | iPSC | 168 | CAR-NK cells with BCMA expression | Unknown | November 24, 2021 | United States |
| NCT05213195 | Recruiting | Phase 1 | Colorectal Cancer | NKG2D | Unknown | 38 | NKG2D CAR-NK | Unknown | December 10, 2021 | China |
| NCT04847466 | Recruiting | Phase 2 | Gastric or Head and Neck Cancer | PD-L1 | Unknown | 55 | Irradiated PD-L1 CAR-NK Cells | 2 × 109 | December 14, 2021 | United States |
| NCT05008575 | Recruiting | Phase 1 | AML | CD33 | Unknown | 27 | Anti-CD33 CA- NK cells | 6 × 108, 12 × 108, 18 × 108 | December 23, 2021 | China |
| NCT05194709 | Recruiting | Early Phase 1 | advanced solid tumors | 5T4) | Unknown | 40 | Anti-5T4 CAR-NK cells | 3 × 109, 4 × 109 | December 30, 2021 | China |
| NCT05410041 | Recruiting | Phase 1 | B-cell malignant tumors | CD19 | Unknown | 15 | CAR-NK-CD19 Cells | 1 × 107/kg, 2 × 107/kg, 3 × 107/kg | May 25, 2022 | China |
| NCT05395052 | Active, not recruiting | Phase 1 | advanced solid tumors | MHC) class I related proteins A (MICA) and B (MICB) | iPSC | 5 | FT536 | Unknown | May 31, 2022 | United States |
| NCT05410717 | Recruiting | Phase I/II | advanced solid tumors (ovarian cancer and others) | CLDN6 | Autologous | 40 | Claudin6 targeting CAR-NK cells | Unknown | June 1, 2022 | China |
| NCT05563545 | Completed | Phase 1 | ALL | CD19 | Unknown | 2 | CAR-NK-CD19 Cells | 1 × 107/kg, 2 × 107/kg, 3 × 107/kg | July 21, 2022 | China |
| NCT05507593 | Recruiting | Phase 1 | SCLC | DLL3 | Unknown | 18 | DLL3-CAR-NK cells | 1 × 107, 1 × 108, 1 × 109, | September 1, 2022 | China |
| NCT05472558 | Recruiting | Phase 1 | DLBCL | CD19 | CB | 48 | Anti-CD19 CAR-NK | 2 × 106/kg, 4 × 106/kg, 8 × 106/kg | September 10, 2022 | China |
| NCT05487651 | Recruiting | Phase 1 | B-cell NHL or leukemia | CD19 | Allogeneic | 36 | CD19.CAR-aNKT cells | 1 × 107/m2, 3 × 107/m2, 1 × 108/m2 | October 1, 2022 | United States |
| NCT05570188 | Withdrawn | Phase I/II | B Cell Hematologic Malignancies | CD19 | PBMC | 0 | Anti-CD19 UCAR-NK cells | 5 × 106/kg, 1 – 2 × 107/kg, 2 – 5 × 107/kg | October 1, 2022 | China |
| NCT05574608 | Recruiting | Early Phase 1 | AML | CD123 | Allogenic | 12 | CD123-CAR-NK cells | 1 × 109, 1 – 2 × 107/kg | October 1, 2022 | China |
| NCT05092451 | Recruiting | Phase I/II | B-Cell LymphomaMDS AML | CD70 | CB | 94 | CAR.70/IL15-transduced CB-NK cells | Unknown | November 1, 2022 | United States |
| NCT05652530 | Recruiting | Early Phase 1 | MM | BCMA | Allogeneic | 19 | BCMA CAR-NK | Unknown | November 13, 2022 | China |
| NCT05645601 | Recruiting | Phase 1 | B-cell Hematologic Malignancies | CD19 | Allogenic | 12 | CD19-CAR-NK | 5 × 106/kg, 2 × 107/kg | December 1, 2022 | China |
| NCT05654038 | Recruiting | Phase I/II | B-cell lymphoblastic Leukemia/Lymphoma | CD19 | Unknown | 30 | Anti-CD19 UCAR-NK cells | 5 – 10 × 106/kg, 1 – 2 × 107/kg,2 – 5 × 107/kg | December 8, 2022 | China |
| NCT05667155 | Recruiting | Phase 1 | DLBCL | CD19 and CD70 | CB | 48 | CB dualCAR-NK19/70 | 2 × 106/kg, 4 × 106/kg, 8 × 106/kg | December 15, 2022 | China |
| NCT05673447 | Not yet recruiting | Early Phase 1 | Diffuse Large B Cell Lymphoma | CD19 | Allogeneic | 12 | anti-CD19 CAR-NK cells | 2 × 108, 1 × 109, 1.5 × 109 | January 1, 2023 | China |
| NCT05842707 | Recruiting | Phase I/II | B-cell lymphoma | CD19/CD70 | CB | 48 | Dual CAR-NK19/70 cell | Unknown | January 18, 2023 | China |
| NCT05528341 | Recruiting | Phase 1 | r/r solid tumors | NKG2D | NK92 cells | 20 | NKG2D-CAR-NK92 cells | 0.5 × 106/kg, 2 × 106/kg | January 26, 2023 | China |
| NCT05336409 | Recruiting | Phase 1 | B-cell malignancies | CD19 | Unknown | 75 | Anti-CD19 CAR-NK cells | Unknown | January 24, 2023 | United States |
| NCT05739227 | Recruiting | Early Phase 1 | r/r B-cell hematologic malignancies | CD19 | Allogeneic | 12 | CD19-CAR-NK cells | Unknown | March 1, 2023 | China |
| NCT05734898 | Recruiting | unknown | r/r AML | NKG2D | Unknown | 30 | NKG2D CAR-NK | Unknown | March 3, 2023 | China |
| NCT05776355 | Recruiting | unknown | Ovarian cancer | NKG2D | Unknown | 18 | NKG2D CAR-NK | Unknown | March 20, 2023 | China |
| NCT05703854 | Recruiting | Phase I/II | renal cell carcinoma mesothelioma or osteosarcoma. | CD70 IL-15 | CB | 50 | CAR.70/IL15-transduced CB-derived NK cells | Unknown | March 29, 2023 | United States |
| NCT05110742 | Not yet recruiting | Phase I/II | Hematological Malignancy | CD5 IL-15 | CB | 48 | CAR.5/IL15-transduced CB-NK cells | 1 × 107, 1 × 108, 1 × 109 | June 30, 2023 | United States |
Abbreviations; PMBC, peripheral blood mononuclear cell; CB, cord blood; NSCLC, non-small-cell lung cancer; CCCR, chimeric costimulatory converting receptor; CLDN6, Claudin 6; AML, acute myeloblastic leukemia; rr, relapsed/refractory; NHL, non-Hodgkin lymphoma, MM, multiple myeloma, MDS, myelodysplastic syndromes; CLL, chronic lymphocytic leukemia; BCMA, B cell maturation antigen; PSMA, prostate-specific membrane antigen; PD-L1, programmed death-ligand 1; ROBO1, roundabout Guidance Receptor 1; 5T4, oncofetal trophoblast glycoprotein.
a Only clinical studies with widely available information on www.clinicaltrials.gov were taken into consideration. ALL, acute lymphocytic leukemia.
1.27. CAR-NK Cell Treatment in the Foreseeable Future and Schedules to Overcome Their Limitations
The major challenges of optimal CAR-NK cell therapy for cancers include immunosuppressive TME, antigen loss, cancer heterogeneity, and impaired homing and distribution of NK cells to tumor sites. These obstacles hamper the persistence and accumulation of NK cells and their cytotoxic mechanisms (79). Several studies have employed innovative solutions to overcome its inevitable limitations.
Unlike CAR-T cells, CAR-NK cells possess the capability to detect tumor antigens and mediate cytotoxic action in both CAR-dependent and CAR-independent ways. Hence, it is possible to design CARs without a co-stimulatory signaling domain and induce intrinsic mechanisms of NK cells for cell-killing rather than CAR-induced direct cytotoxicity. Engineering NK cells to express homing-promoting target factors such as chemokines and adhesion molecules is another non-signaling CAR construct. Similarly, the insertion of additional co-stimulatory domains, such as DNAX-activation proteins 10 or 12 (DAP-10 or DAP-12) and NK-specific 2B4, into the CAR structure could amplify the immunoregulatory impact of CAR-NK cells (57). Another potential method involves co-modifying CAR constructs with cytokines, antibodies, and proteases to improve the proliferation, trafficking, persistence, and deep tumor penetration of NK cells.
2. Conclusions
The emergence of "off-the-shelf" CAR-NK cells has opened a new promising window for clinical use. With the ability to tackle these challenges, this product presents a safe and available CAR-T cell alternative for immunotherapy in different types of cancers, even in HLA-unmatched patients. However, some limitations, including the response of the recipient’s immune system and the short lifespan of the injected cells in the body, as well as the immunosuppressive TME, limit their potential applications.