1. Context
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive fibrous interstitial lung disease characterized by uncontrolled proliferation and differentiation of myofibroblasts, excessive deposition of collagen in the lung interstitial and alveolar lumen, and eventually leading to respiratory failure. The etiology and pathogenesis of IPF have not been fully elucidated. The clinical manifestations are mainly cough and shortness of breath after activity, and morphological manifestations are mainly histopathological patterns of progressive lung scarring and usual interstitial pneumonia (UIP). The median survival time is 3 to 5 years (1). Idiopathic pulmonary fibrosis mainly occurs in the elderly population and is limited to the lungs, and it mostly occurs in elderly men with a history of smoking (2). Meanwhile, the incidence of chronic obstructive pulmonary disease, pulmonary hypertension, coronary artery disease, and lung cancer (LC) (3) in IPF patients is higher than that of healthy peers.
Some studies have shown that patients with IPF are at high risk for LC, especially older men who smoke. Studies on IPF complications over the years have found that smoking history, advanced age, male sex, and emphysema may be important risk factors for LC in patients with IPF. In another study involving 1143 LC patients, it was reported that combined pulmonary fibrosis and emphysema (CPFE) can increase the risk of LC in patients. Approximately 8.9% of LC patients were found to have CPFE, while 1.3% of patients were found to have isolated IPF (4). Based on the current understanding of the pathogenesis of IPF and LC diseases, it has been found that there are many common pathogenic mechanisms between IPF and LC, such as excessive activation and proliferation of myofibroblasts (IPF) and cancer-associated fibroblasts (CAF), changes in the expression level of growth factors, and common gene mutations. These will eventually lead to the occurrence and progression of IPF and LC diseases. Thus, there is a closed relationship between IPF and LC (5). Existing studies have shown that IPF complicated with LC is not accidental. This article will review the correlation between IPF and LC in etiology, pathogenesis, biomarkers, and therapeutic drugs.
2. Evidence Acquisition
This article reviews the epidemiology, clinical diagnosis, pathological mechanisms, potential targets, and current clinical treatment status of pulmonary fibrosis combined with LC. For the literature review, we employed a standard search strategy, including querying the PubMed online database using terms such as “pulmonary fibrosis combined with lung cancer” and “therapy”. The reference lists of each article were thoroughly examined to find additional relevant articles. The identified articles underwent independent full-text review.
3. Results
3.1. Epidemiology and Clinical Diagnosis of IPF Combined with Lung Cancer (IPF-LC)
3.1.1 Epidemiology
Idiopathic pulmonary fibrosis is an independent risk factor for LC, according to the 5 criteria for precancerous lesions established by the National Cancer Institute in the United States, IPF is also considered a precancerous lesion (6). Some studies have shown that IPF patients have a high risk of LC (4), which is 5 times higher than the general population. It has been reported that the probability of LC in IPF patients is 3% to 45.7%, and in Japan, 11% of IPF patients die of LC (5). A retrospective study on the cumulative incidence of LC in IPF showed that the incidence of LC was 3.3%, 15.4%, and 54.7% in the 1st, 5th, and 10th years of follow-up for IPF patients, respectively (1). Furthermore, data from a follow-up survey study of IPF patients shows that the cumulative incidence of LC in IPF patients at 1 year and 3 years is 41% and 82%, respectively, with significantly lower survival rates in IPF patients with LC compared to those without LC (7). Therefore, there is a closed relationship between IPF and LC. In addition, most patients with IPF combined with lung cancer (IPF-LC) are male and have a long history of smoking, the incidence of LC is 9 times higher in men compared to women and in smokers compared to non-smokers (8). In terms of pathological classification, lung squamous cell carcinoma is the most common (about 48.4%) in IPF-LC, followed by adenocarcinoma, large cell carcinoma, and small cell carcinoma (9). Some studies have reported that smoking, age, gender, emphysema, and other factors will increase the risk of LC in IPF patients (10).
3.1.2 Clinical Diagnosis
3.1.2.1 Clinical Symptoms
The most common symptoms in patients with IPF are cough, dyspnea, and finger clattering, while chest pain, hemoptysis, and emaciation are more common in patients IPF-LC. Multivariate regression analysis of one study showed that chest pain (P = 0.001) and hemoptysis (P = 0.000) were suggestive of IPF-LC. The main clinical symptoms of IPF-LC patients are dry cough, shortness of breath, and hemoptysis, which suggest the possibility of LC in IPF patients (11).
3.2. Common Pathogenesis of IPF Combined with Lung Cancer (IPF-LC)
3.2.1 Gene Mutation
Although the pathogenesis of IPF remains unclear, there is increasing evidence that genetic variation can explain the development of the disease. The development of LC is the result of the accumulation of multiple somatic gene mutations (9). Studies have shown that functional mutations of surfactant-related genes are associated with the pathogenesis of both IPF and LC. The imbalance between the expression of proto-oncogenes and tumor suppressor genes is the molecular basis of carcinogenesis, and there is also an imbalance between proto-oncogenes and tumor suppressor genes in IPF. The mutation of the p53 gene caused by chronic DNA damage in IPF patients may promote the occurrence of LC (12).
3.2.2 Epigenetics
Epigenetic alterations are thought to be the common causative factor between IPF and LC, and genome-wide methylation analysis showed that IPF and LC samples had similar methylation profiles. Besides, it has also been suggested that aberrant expression of certain noncoding RNAs plays a role in both fibrosis and carcinogenesis. For example, the expression of MicroRNA (miR)-21 is highly up-regulated in IPF patients, and the increased expression of miR-21 is associated with the low survival rate and recurrence of non-small cell lung cancer (NSCLC) patients (13).
3.2.3 Signaling Pathway
Idiopathic pulmonary fibrosis and LC are characterized by aberrant activation of major signal transduction and developmental pathways. Canonical Wnt/β-catenin pathway (Canonical Wnt/β-catenin pathway) is dysregulated by over-expression of its target genes, such as those encoding cyclin D1 and matrix metalloproteinase (MMP)-7. It causes cancer metaplasia, and lung remodeling and regulates the epithelial-mesenchymal transition (EMT) process (14). Activation of the phosphoinositol 3-kinase/protein kinase B (PI3K/AKT) signaling pathway is associated with fibroproliferative diseases and cancer invasion. PI3K has been shown to activate profibrotic mediators such as transforming growth factor-β1 (TGF-β1) and platelet-derived growth factor (PDGF). Therefore, inhibition of PI3K has been proposed for the treatment of IPF and LC (15). Sonic Hedgehog, Shh (Shh) signaling pathway is abnormally activated in bronchial epithelial cells around honeycomb cysts and increases the occurrence of fibroblast apoptosis, EMT, tumor growth, metastasis, and chemotherapy resistance (15). Tyrosine kinase (TK) signal transduction is one of the key regulatory pathways involved in cell metabolism, growth, differentiation, adhesion, cell death, and angiogenesis. Dysregulated TK can promote the development and progression of tumors, cardiovascular, and fibrotic diseases (16). Tumor cells use PD-1/PD-L1 as a checkpoint to maintain systemic immune homeostasis and self-tolerance to evade the surveillance of the immune system. PD-1 was significantly up-regulated in lymphocytes of patients with IPF, and pulmonary fibroblasts and CD4+T cells overexpressing PD-L1 promoted fibrosis progression. Blocking PD-1/PD-L1 with specific monoclonal antibodies has been established as a first-line treatment for advanced NSCLC (17). Moreover, studies have shown that inhibition of PD-L1 can attenuate experimental pulmonary fibrosis. Therefore, the PD-1/PD-L1 pathway may be the potential common pathogenic mechanism of IPF-LC (18).
3.3. The Treatment Status of IPF Combined with Lung Cancer (IPF-LC)Patients
At present, there is no unified consensus on the management and treatment of patients with IPF-LC. The reasonable management and treatment of patients with IPF-LC are a dilemma facing clinical practice. At present, single treatment methods such as surgery, radiotherapy, chemotherapy, and targeted therapy may lead to acute exacerbation of IPF (AE-IPF). Combination therapy of multiple means or the treatment of anti-cancer and anti-fibrosis drugs will be the main direction of the treatment of IPF-LC patients in the future.
3.3.1 Non-drug Therapy for IPF Combined with Lung Cancer (IPF-LC)
3.3.1.1 Surgical Treatment
Surgical resection is the main treatment for early LC, but AE-IPF is a life-threatening complication after pneumonectomy in patients with IPF-LC, and the mortality is high. A retrospective study of 350 patients with early-stage NSCLC undergoing pneumonectomy showed that the 5-year survival rate for LC patients with IPF was significantly lower than that of patients without IPF (54.2% vs 88.3%) (19). It has been reported that among IPF patients undergoing LC surgery, the incidence of AE-IPF in postoperative patients is about 20%, and the associated mortality is about 50% (20). Although surgical treatment is effective for early-stage LC, available data show that LC patients with IPF have higher pneumonectomy-related mortality. Therefore, whether to perform surgical treatment for patients with IPF-LC needs comprehensive consideration. An international survey on the diagnosis and management of IPF-LC showed that most doctors believed that surgery was acceptable for early operable LC patients with mild-to-moderate IPF (78.2%), while for operable LC patients with severe IPF, the consent rate for surgery decreased significantly (21.4%) (21).
3.3.1.2 Radiotherapy
IPF is associated with severe and fatal complications in LC patients after radiotherapy. A retrospective study reported that 18.2% of patients with early-stage non-small cell LC complicated with IPF who received radical radiotherapy had radiation-related death, and AE-IPF caused by radiotherapy was the main cause of death (22). Basis of pulmonary disease affects LC after radiotherapy of lung toxicity, in a study of LC patients with underlying lung diseases of proton beam therapy of small retrospective study, 15 patients with IPF-LC proton beam therapy in 20% of patients after lung toxicity level 3, 13.3% of the patients appeared lung toxicity level 5, but for a year overall survival in patients with no obvious influence, there were no treatment-related deaths after proton beam therapy (23). Compared with the incidence of grade 5 pulmonary toxicity in patients with interstitial lung disease after radiotherapy (26.8 - 53.8%), proton beam therapy has a lower incidence of grade 5 pulmonary toxicity (24). Therefore, proton beam therapy may be a useful treatment modality for early-stage LC patients with poor pulmonary function or pulmonary fibrosis. Pneumothorax is one of the leading causes of death in patients with radiotherapy, and about 40% of IPF patients often have emphysema, which will be a factor in whether to consider radiotherapy in patients with IPF-LC. Despite this, nearly half of the physicians considered stereotactic radiation therapy to be the primary intervention for patients with severe IPF and operable IPF-LC. However, current data of radiotherapy IPF-LC are still very few, and more clinical data are needed for retrospective analysis.
3.3.2 Drug Therapy for IPF Combined with Lung Cancer (IPF-LC)
3.3.2.1 Chemotherapy
Acute exacerbation of IPF is a fatal and uncommon complication of chemotherapy in patients with IPF-LC. After chemotherapy, UIP symptoms and reduced FVC values may occur; 63% of IPF LC patients receiving chemotherapy can have a pulmonary infection, neutropenia, respiratory insufficiency, and cardiovascular complications (19). Docetaxel monotherapy or docetaxel-containing regimen has a low overall response and disease control rate to IPF-LC, and the treatment outcome is poor. Pemetrexed treatment can cause pulmonary toxicity, and the risk of interstitial lung disease in patients rises to 12.0%, and the risk of UIP in patients is as high as 16.7% (25). In addition, a retrospective study reported that carboplatin combined with paclitaxel is effective in IPF patients with advanced non-small cell LC. The risk of AE IPF is still high and cannot be ignored (26). At present, there is little data about the best chemotherapy regimen for patients with IPF-LC. In general, the single therapy of chemotherapy has a poor treatment effect on the disease and a high pulmonary toxicity rate, while chemotherapy combined with anti-pulmonary fibrosis drugs may be a more effective treatment.
3.3.2.2 Immunotherapy
Because of the similarity between the disease mechanisms of IPF and LC, a large number of approved NSCLC therapeutic agents have been identified for potential use in the treatment of pulmonary fibrosis. For example, nivolumab is a novel immunomodulator as a blocking antibody to PD-L1. A study of a patient with IPF in lung adenocarcinoma showed an effective and sustained pulmonary response after nivolumab treatment without any sign of IPF deterioration. Other viable therapeutic candidates for IPF-LC include vantituzumab, which interferes with Wnt signaling and has been tested in phase I trials for NSCLC (preclinical studies of Wnt pathway inhibition have also been performed in pulmonary fibrosis models). Lovapituzumab interferes with the Notch signaling pathway and is currently approved for SCLC. At present, artesunate has been found to improve pulmonary fibrosis by inhibiting the Notch signaling pathway in the rat bleomycin model; so, rovapituzumab may also have therapeutic potential for IPF and IPF-LC (27). Given similar pathologic mechanisms of IPF and LC, the use of immunomodulators in patients with high PD1/PD-L1 expression levels will be important, but the occurrence of interstitial pneumonia associated with antibody therapy still needs to be considered.
3.3.2.3 Antifibrotic Therapy
Pirfenidone and nintedanib are two antifibrotic drugs approved for IPF, which are also suggested to prolong the survival time and reduce the incidence of LC in patients with IPF-LC (28). Many studies have confirmed that pirfenidone can stabilize pulmonary function parameters and delay the decline of pulmonary function in IPF patients, and it is well tolerated in most patients. At the same time, pirfenidone can show anti-tumor and delay the progression of IPF-LC by activating the apoptosis pathway of tumor-related fibroblasts and inhibiting a variety of TK that contribute to tumor angiogenesis and fibrosis (29). Nintedanib is a multiplex TK inhibitor that is currently approved for the treatment of IPF. In a retrospective analysis of a 78-year-old patient with stage IIIB lung adenocarcinoma complicated with IPF, nintedanib was reported to be effective in the treatment of this patient. In addition, two Japanese case reports also reported that patients with IPF-LC treated with nintedanib had pulmonary mass resolution or stable pulmonary nodules and fibrosis without progression for 9 months. However, there is still a lack of research on nintedanib in IPF-LC. Based on the effect of nintedanib on IPF and LC, nintedanib may be a potential drug for the treatment of IPF-LC (23).
3.3.2.4 Combination Therapy
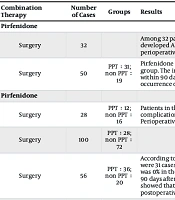
Acute exacerbation of IPF is the most lethal complication in IPF-LC patients undergoing surgery, chemotherapy, and targeted therapy, with a high mortality rate. If there is a strategy to reduce AE-IPF, patients can be treated more safely and reasonably and the prognosis of patients can be improved (30). Many studies have shown that perioperative pirfenidone treatment can reduce the incidence of AE-IPF after LC surgery. Pirfenidone combined with chemotherapy or immune checkpoint inhibitors (ICI) can reduce AE-IPF associated with LC treatment, which may become a treatment option for patients with IPF-LC. Clinical evidence has shown that nintedanib combined with chemotherapy or ICI monoclonal antibody therapy can alleviate IPF-LC, improve event-free survival (EFS) and overall survival QI (OS), and prevent the occurrence of AE-IPF (Table 1 for relevant clinical studies on combination therapy). According to the existing clinical studies, most doctors suggest that pulmonary fibrosis therapy should be maintained during the diagnosis and treatment of LC , and the clinical benefits of anti-pulmonary fibrosis combined with anticancer therapy outweigh the risk of adverse reactions.
| Combination Therapy | Number of Cases | Groups | Results | Time |
|---|---|---|---|---|
| Pirfenidone | ||||
| Surgery | 32 | Among 32 patients with confirmed IPF-LC treated with pirfenidone for more than two weeks before surgery, 1 patient developed AE IPF within 30 days after surgery, with a non-AE rate of 96.9%. This single-arm phase 2 study showed that perioperative pirfenidone treatment can reduce the incidence of AE IPF after LC surgery. | 2016 (20) | |
| Surgery | 50 | PPT:31; non PPT:19 | Pirfenidone was administered orally from 4 weeks before the operation to 4 weeks after the operation in the PPT group. The incidence of AE-IPF in the PPT group and the non-PPT group was 0%/10.5% within 30 days and 3.2%/21.1% within 90 days, respectively. The results showed that perioperative pirfenidone treatment can prevent the occurrence of AE-IPF after LC surgery. | 2016 (29) |
| Pirfenidone | ||||
| Surgery | 28 | PPT:12; non PPT:16 | Patients in the PPT group were treated with pirfenidone 2 - 5 weeks before surgery. No serious pirfenidone-related complications or IPF-related events occurred in the PPT group, while 6 control patients developed AE IPF. Perioperative pirfenidone is a feasible treatment for patients with IPF-LC and may prevent the occurrence of AE IPF. | 2014 (31) |
| Surgery | 100 | PPT:28; non PPT:72 | 2020 (32) | |
| Surgery | 56 | PPT:36; non PPT:20 | According to the JACS score, there were 6 low-risk patients and 22 medium-high-risk patients in the PPT group. There were 31 cases of low risk and 41 cases of medium to high risk in the non-PPT group. In low-risk patients, the AE rate was 0% in the PPT group and 6.5% in the non-PPT group at 30 and 90 days after surgery. The incidence of AE at 30 and 90 days after operation was 4.5% in the PPT group and 19.5% and 24.4% in the non-PPT group, respectively. The results showed that PPT is an effective and feasible prophylactic treatment for IPF-LC, which can reduce the occurrence of postoperative AE. | 2020 (33) |
| Chemotherapy | 14 | PPT:12; non PPT:16 | There was no difference in age, gender, smoking history, respiratory function, or surgery between the PPT group and the non-PPT group. There were 3 patients in the PPT group and 4 patients in the non-PPT group with AE. There was no significant difference between the two groups, but the time interval of AE in the PPT group was significantly longer, and the postoperative mortality in the PPT group was significantly lower. The results showed that perioperative pirfenidone could not significantly prevent postoperative AE-IPF, but may reduce the mortality of IPF-LC patients. | 2020 (34) |
| Pirfenidone | ||||
| Surgery | 28 | PPT:12; non PPT:16 | Patients in the PPT group were treated with pirfenidone 2 - 5 weeks before surgery. No serious pirfenidone-related complications or IPF-related events occurred in the PPT group, while 6 control patients developed AE IPF. Perioperative pirfenidone is a feasible treatment for patients with IPF-LC and may prevent the occurrence of AE IPF. | 2014 (31) |
| Surgery | 100 | PPT:28; non PPT:72 | According to the JACS score, there were 6 low-risk patients and 22 medium-high-risk patients in the PPT group. There were 31 cases of low risk and 41 cases of medium to high risk in the non-PPT group. In low-risk patients, the AE rate was 0% in the PPT group and 6.5% in the non-PPT group at 30 and 90 days after surgery. The incidence of AE at 30 and 90 days after operation was 4.5% in the PPT group and 19.5% and 24.4% in the non-PPT group, respectively. The results showed that PPT is an effective and feasible prophylactic treatment for IPF-LC, which can reduce the occurrence of postoperative AE. | 2020 (32) |
| Nintedanib | . | |||
| Chemotherapy | 243 | Patients with IPF-LC who received nidanib plus carboplatin and albumin-bound paclitaxel improved overall survival in patients with non-squamous cell LC and GAP1 stage compared with those who did not receive nidanib, with only 2.9% of patients developing AE-IPF during treatment. | 2022 (35) | |
| Artemizu-mab | 1 | A patient with IPF and lung squamous cell carcinoma developed associated pneumonia after pimplumab treatment, which improved after prednisolone treatment, and developed pneumonia after the progression of LC, which was treated with atezumab and developed pneumonia. The patient remained stable after 3 weeks of increased prednisolone plus ganidanib. This case suggests that treatment with ICI in combination with nidanib may prevent drug-induced pneumonia or AE-IPF | 2019 (30) |
Abbreviation: PPT, perioperative pirfenidone treatment; perioperative pirfenidone treatment group; LC, lung cancer; IPF, Idiopathic pulmonary fibrosis.
4. Conclusions
IPF combined with lung cancer is becoming more and more common in clinical, but there is no effective treatment, which seriously affects the quality of life and survival time of IPF-LC, and the prognosis is very poor. Due to the propensity of current treatment modalities to induce acute exacerbations of pulmonary fibrosis, the treatment of IPF-LC presents challenges. To improve patient outcomes, it is necessary to adjust the current LC screening guidelines for patients with pulmonary fibrosis, utilizing earlier and more reliable cancer detection methods to avoid delays in treatment (6). Additionally, through an in-depth understanding of the clinical characteristics and pathogenesis of IPF-LC and the discovery of its potential targets, combination therapy may be an effective strategy for the treatment of IPF-LC in the future. However, there is no effective preclinical evaluation method for IPF-LC treatment; so, it is urgent to explore appropriate animal models and more clinical studies in the future to find new strategies that may be safe and effective for IPF-LC.