1. Background
The accumulation of antigen-presenting dendritic cells in granulomatous lesions can lead to single system langerhans cell histiocytosis (SS-LCH) or multi-system LCH (MS-LCH) (1) which are rare malignancies that can affect any age ranges of children (1, 2). the age-specific rate (ASR) of LCH in patients younger than 15 years old is 4 to 9 per 1 000 000 children (3, 4).
As a result of being heterogenous, this malignancy can have multiple clinical symptoms, which lead to various prognoses and outcomes (4). A report in 2016 revealed that severe types of LCH are mainly diagnosed in cases less than 2 years old which can result in poor prognosis (5). Routinely, the main treatment modality in pediatric LCH is chemotherapy which differs based on the new cases or recurrent LCH patients (3). In 2015, a study was published that confirmed hematopoietic stem cell transplantation in patients with recurrent LCH (6) which could access 70% of overall survival in that patients.
Iran as a low-middle-income country has a noticeable rate of pediatric malignancies in where the prevalence of childhood LCH could be worthwhile for further clinical research. By designing the clinical trials through these cases and evaluating the modern therapies, the cure rate could increase and as a result, mortality, poor outcomes, and prognoses will decrease. By the way, there could be some limitations beyond this idea, that relate to the rare reports of Iranian pediatric patients with LCH. The majority of published papers from Iran are review articles, which considered childhood LCH and scare reports around epidemiology from Iranian pediatric malignancies’ single centers. Finally, these limitations turned on the light of designing the current project for considering the care and management of Iranian childhood LCH in a referral pediatric cancer center. Regarding this idea, the survival rates of these cases were evaluated too.
2. Methods
2.1. Study Design and Patients
The study was conducted as a cross-sectional retrospective hospital-based study which included all of the patients younger than 15 years old who had been diagnosed as new cases with LCH from 2007 to 2019. The exclusion criteria were defined for those patients who lost the therapy during the courses and left their treatment for unknown reasons. The follow-up of enrolled patients was done in 2022 to estimate the survival rates.
2.2. Data Gathering
The ethical committee of the hospital approved the designed unique questionnaire for gathering patients' data based on demographic information, pathology reports, treatment modalities, and status updates. The gathered data was entered into SPSS software version 23 for further analysis.
2.3. Patients’ Risk Stratification
Patients were categorized into 3 risk groups based on below criteria (7, 8):
- Multisystem high-risk patients: Who had involvement of one or more risk organs like the hematopoietic system, liver, spleen, or lungs
- Multisystem low-risk patients: With multiple organs involved but without involvement of risk organs
- Single system multifocal bone disease (moderate risk): Or localized special site involvement which included patients with multifocal bone disease (lesions in two or more different bones) or with localized special site involvement (lesions with intracranial soft-tissue extension or vertebral lesions with intra-spinal soft-tissue extension)
2.4. Patients’ Systemic Chemotherapy
The initial phase of systemic chemotherapy for all of the risk groups started with (9):
- Prednisolone (Pred) for 14 days in 2 weeks followed by taper for 2 weeks.
- Vinblastine (VBL) on the first day of the week for 6 weeks.
- Methotrexate (MTX) on the first day of weeks 1, 3, and 5.
- Leucovorin (Leuc) for 3 days started on the second day of weeks 1, 3 and 5.
At the end of the initial phase, the patient was evaluated and the next phase was continued based on their risk groups as below: (1) group 1: Received the course of VBL, 6-Mercaptopurine (6-MP), Pred (for 5 days), and MTX (weekly). Patients of this group were administered 16 courses, which were repeated every 21 days; (2) groups 2 and 3: Received the course Pred (for 3 days) and VBL per week, which continued for 6 weeks.
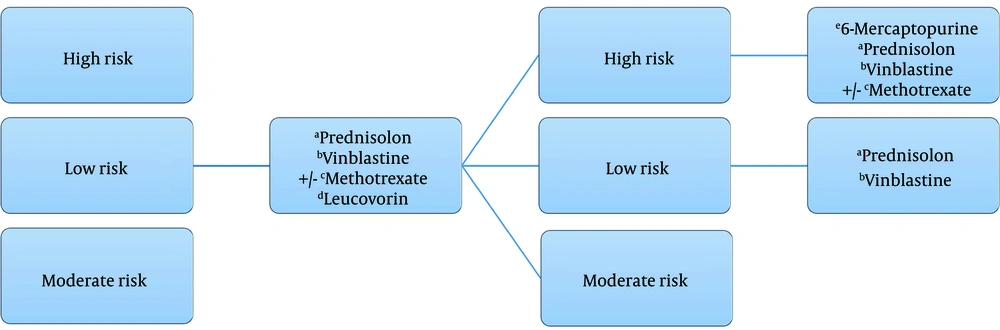
Figure 1 shows the schematic view of treatment in considered cases and the dosage of chemotherapy agents.
2.5. Data Analysis
Analyses were done by SPSS software version 25 for parametric and non-parametric data. Kolmogorov-Smirnov was used for evaluating the normality of variables also, Fisher test and chi-square tests were used for the relation between variables, in addition, ANOVA was used for parametric variables.
The overall survival rate was defined as the duration of the time between the date of diagnosis and the date of last status in patients. The last status was confirmed as last visit, last phone call, or date of death. The relapse and death were considered as events and also the event-free survival was estimated based on the time duration from diagnosis to the first event in patients. These rates were calculated by Kaplan-Meyer analysis.
2.6. Ethical Committee
The ethical committee of AJA University of Medical Sciences, Tehran, Iran with the ethical committee code IR.AJAUMS.REC.1401.063 approved the designed project
3. Results
3.1. Patients
During 12 years of evaluation, 32 new cases with approved pediatric LCH (patients younger than 15 years old) were administered who were 17 (53.1%) and 15 (46.9%) female and male, respectively (male to female ratio: 0.88). Out of those cases, 18 children were treated as high risk and the others were related to moderate (n = 9) and low (n = 5) risk groups.
The frequency of males to females was higher only in the high-risk group (M/F: 10/8) while it was vice versa in low and moderate-risk groups (M/F of low-risk patients: 2/3; M/F of moderate-risk group: 3/6). The mean age of total patients was 5.1 ± 0.7 years old (age range 1 - 14 years old), and there was not any significant relation between the risk groups and patients’ age (P-value > 0.05).
The chief complaints or symptoms of patients that were the cause of referee to the clinic were bone pain (in 62.5% of cases) and eyelid swelling (28.1% of patients). Analyses of data based on the site of disease revealed that the most common involvement was in the skeleton (87.5% of total cases) followed by skin (21.9% total), in addition, the rate of hematopoietic system involvement was the same in lymph nodes, and the less site related to central nervous system (Table 1). Clinical manifestations showed that only 5 patients had hepato-splenomegaly.
| Variables | High Risk | Low Risk | Moderate Risk | |||
|---|---|---|---|---|---|---|
| Male (n = 10) | Female (n = 8) | Male (n = 2) | Female (n = 3) | Male (n = 3) | Female (n = 6) | |
| Mean age (y) (Min-Max) | 6.3 ± 1.4 (2 - 14) | 5.2 ± 1.5 (1 - 13) | 5.5 ± 3.5 (2 - 9) | 5 ± 3.1 (1 - 11) | 2 ± 1 (1 - 4) | 4.5 ± 1.5 (1 - 9) |
| Site of disease (n) | ||||||
| Skeleton | 9 | 7 | 1 | 2 | 3 | 6 |
| Skin | 1 | 2 | 1 | 2 | 0 | 1 |
| Hematopoietic system | 0 | 0 | 0 | 2 | 2 | 0 |
| Lymph nodes | 0 | 1 | 0 | 1 | 0 | 2 |
| Central nervous system | 0 | 0 | 0 | 0 | 0 | 3 |
| Chief complaint (n) | ||||||
| Mouth mucositis | 1 | 0 | 1 | 0 | 0 | 0 |
| Bone pain | 6 | 5 | 1 | 3 | 2 | 3 |
| Eyelid swelling | 4 | 4 | 0 | 0 | 0 | 1 |
| Ptosis | 2 | 1 | 1 | 0 | 0 | 1 |
| Hepato and splenomegaly | 0 | 0 | 2 | 2 | 1 | 0 |
3.2. Patients’ Treatment Modalities
All of the patients had chemotherapy protocol according to the risk groups (Figure 1). The type of surgery based on diagnostic biopsy, total or partial resection are categorized in Table 2. There was only one female low-risk case who did not have any surgery.
| Variables | High Risk | Low Risk | Moderate Risk | |||
|---|---|---|---|---|---|---|
| Male (n = 10) | Female (n = 8) | Male (n = 2) | Female (n = 3) | Male (n = 3) | Female (n = 6) | |
| Surgery (n) | ||||||
| Biopsy | 3 | 4 | 1 | 2 | 2 | 1 |
| Total resection | 6 | 4 | 1 | - | 1 | 5 |
| Partial resection | 1 | - | - | - | - | - |
| Radiotherapy | 0 | 1 | 0 | 0 | 2 | 0 |
| Relapse | 2 | 2 | 0 | 0 | 0 | 1 |
Only 3 cases had radiotherapy:
(1) A 15-year-old male from a moderate risk group with skeleton and hematopoietic system involvement who referred with bone pain.
(2) A 15-year-old male from a moderate risk group with skeleton involvement and had hepato and splenomegaly at the time of diagnosis.
(3) A 13-year-old female from high-risk group with skin involvement and referred with eyelid swelling.
All of those three cases were alive at the time of writing this paper, and unfortunately, there was not enough data about the dose and site of radiotherapy for them.
Based on the data in Table 2, only 5 cases had relapse and the mean time of recurrence (from diagnosis to the first relapse) was 2.6 ± 0.8 years (range 1 to 5 years).
3.3. Survival Rates
Unfortunately, only 2 cases died who were:
(1) A 7-year-old male from low-risk group who had mouth mucositis and hepatosplenomegaly with skin involvement (dead after 30 months following diagnosis).
(2) An 11-year-old female from the low-risk group who referred with bone pain and hepatosplenomegaly and had skin involvement (dead after 3 months following diagnosis).
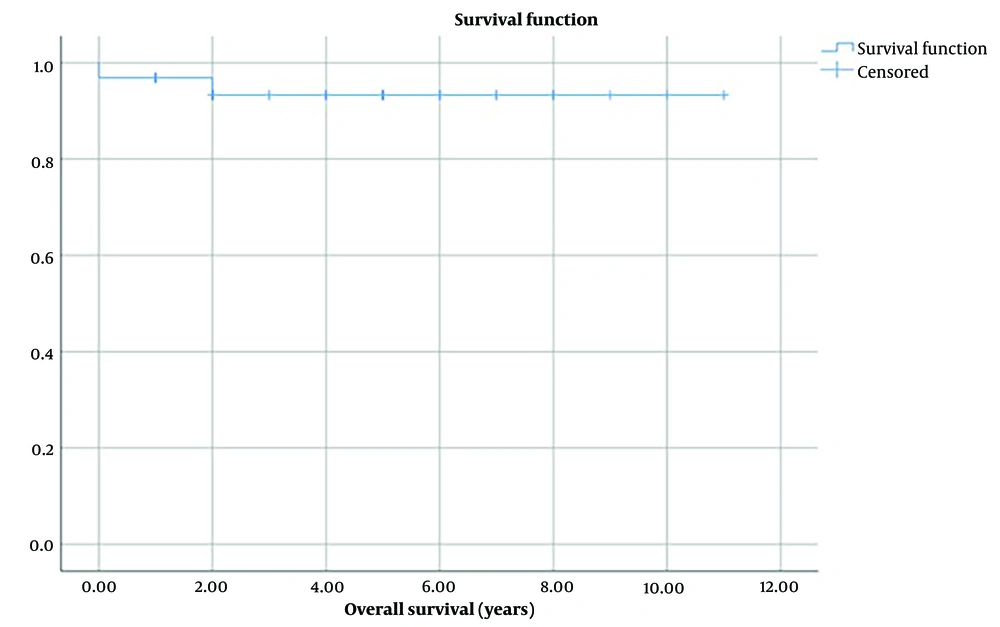
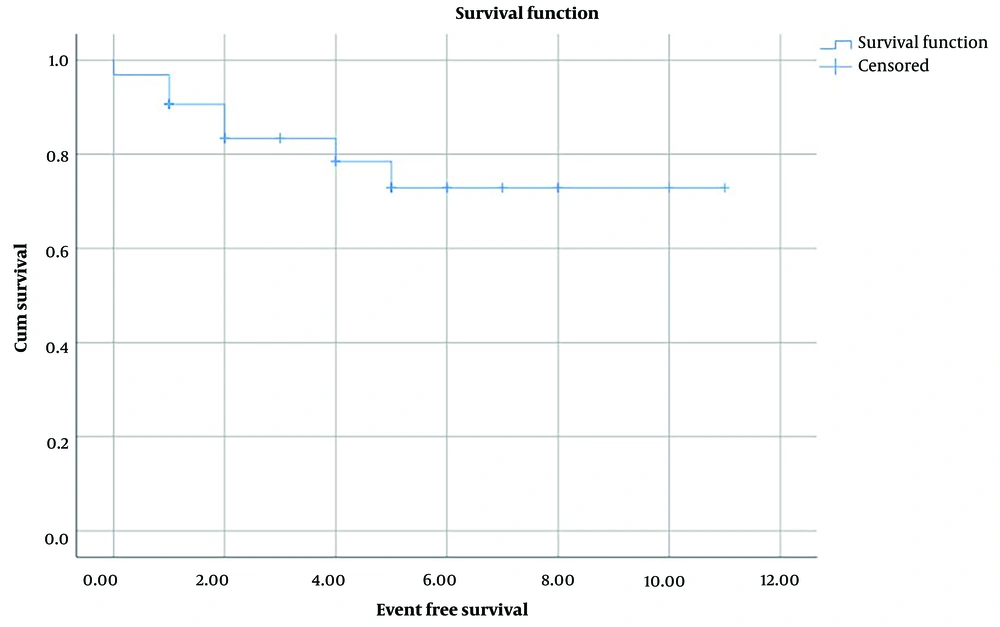
The survival rate from diagnosis to the last update of patients was from 3 months to 11 years (mean time 4.5 ± 0.5 years). The 5-year overall survival rate in enrolled patients was 93.3% ± o.o5 (Figure 2). The event-free time was from 3 months to 11 years with the meantime of 4 ± 0.5 years, and also the 5-year event-free survival rate was 72.9% ± 0.09 (Figure 3).
4. Discussion
Langerhans cell histiocytosis (LCH) is due to the accumulation of pathologic Langerhans cells in the bones, skin, bone marrow, brain, etc. Furthermore, heterogeneous presentationscan present both in children and adults (2). In 2020, a review study confirmed that the incidence rate of LCH in children younger than 15 years old ranged from 2.6 to 8.9 cases per million children (the median age at diagnosis: 3 years old) (10). The incidence rate of this malignancy is similar to pediatric Hodgkin Lymphoma (11).
According to the design of this study, the demographic characteristics and survival rates of Iranian pediatric patients with LCH were considered. The main idea of conducting this project was related to the rarity of published data about the mentioned patients. The gathered data from 32 patients with LCH revealed a slight increase in female patients with a mean age of 5 years old. At that time, the majority of patients were high risk, otherwise, there was not any death in that risk group, while there were only 2 deaths from low-risk group patients. The chief complaint of bone pain and the skeleton site involvement was common throughout the patients, and finally, the 5-year overall survival and event-free survival rates (93.3% and 72.9%) were reasonable based on patients’ therapy.
In 2023, based on literature reviews, Kemps announced that the severe forms of LCH relate to patients younger than 2 years old who have involvements of the hematopoietic system, liver, and spleen (12). The results of this study confirmed this point, as two cases who died had involvement of liver and spleen, but there were not any patients younger than 2 years old with LCH. Moreover, that study (12) highlighted the low mortality rate in pediatric patients with LCH alongside a significant rate of relapse. Our present study findings corroborate these observation, as we also noted a low rate of deaths and nearly 16% of patients experiencing relapsed.
Between 2000 and 2004 in France, 258 cases with LCH younger than 15 years were evaluated. The male-to-female ratio was 1.2 and the most involved organs were bone and skin. That scientific group confirmed the 2-year overall survival rate of 99% in considered patients (13). In comparison with our data, the female ratio of enrolled patients in the presented study was more than France's study, and according to figure 2, the 2-year survival rate of cases was the same as 5-years survival rate (93.3%) that is lower than France's study.
In 2022, a report was published from 6 years of evaluation (from 2013 to 2019) of registered patients with LCH in London. In total, 658 patients enrolled in that study of which 49% of them were children (younger than 15 years old) (14). Other information from that study relates to the incidence and prevalence of cases that could be on behalf of our limitations through the registry of pediatric cancers in Iran. In this regard, all of the reports from Iranian pediatric LCH are from hospital-based studies with a limited considered case.
Another study that considered 91 pediatric patients with multifocal LCH reported a 5-year survival rate of 100% for their single-system multisite patients (n = 32 cases) and 94% for multisystem patients (n = 59 patients) (15). That study was designed and performed in 2006 in Japan, which that means currently improving in their survival rate is inevitable.
Literature reviews have confirmed that one of the most critical considerations in pediatric patients with LCH is the role of genetic factors, particularly those associated with treatment response.
The worst comment is that setting up molecular evaluations in Iran is a hardship because of sanctions, and in this regard, the current research around these cases already relate to epidemiological data and survival rates of these cases. Another effective point is that there is no national pediatric cancer registry in Iran, in that way all of the reports are hospital-based studies that contain a limited number of cases. Finally, the treatment protocols in Iran improve gradually, and administrating modern chemical agents is complicated. All of the above-mentioned factors can negatively effect on the data and results of this study, however, the most important point is that this 5-year survival rate is desirable for considered cases.
4.1. Conclusions
In summary and based on the results, the suggestion is to provide a national registry for pediatric LCH followed by designing future projects around affective genes on the treatment response of mentioned patients. In that way, we can improve the survival rate of these patients and decrease mortality.