1. Background
Recent advancements in the management of non-small cell lung cancer (NSCLC) have marked significant progress, particularly with the emergence of targeted therapies and immunotherapies (1, 2). Targeted therapies, directed at specific molecular aberrations, have notably improved patient outcomes for distinct NSCLC subtypes. Additionally, immune checkpoint inhibitors have shown efficacy in enhancing the immune system's response against cancer cells (3, 4). Despite these advances, challenges such as resistance to targeted therapies and variable response rates among patient subsets persist. Addressing these obstacles, enhancing patient selection precision, and pioneering new therapeutic strategies remain critical endeavors in the quest to optimize and personalize treatment regimens for NSCLC.
Lycopene, a naturally occurring carotenoid pigment abundantly found in tomatoes and other red fruits, has garnered significant attention in recent years due to its potential pharmacological effects and health benefits (5-7). As a potent antioxidant, lycopene has been extensively studied for its role in combating oxidative stress, a key factor implicated in numerous chronic diseases. Recent research has focused on elucidating the molecular mechanisms underlying lycopene's antioxidant properties. Studies have demonstrated its ability to neutralize reactive oxygen species (ROS) and inhibit lipid peroxidation, thereby protecting cells from oxidative damage (8, 9). These insights underscore the potential of lycopene in the management of oxidative stress-related conditions, encompassing cardiovascular diseases and certain forms of cancer. Additionally, research into lycopene's anti-inflammatory effects has identified its ability to influence inflammatory cascades. This has been demonstrated through the modulation of critical inflammatory biomarkers, positioning lycopene as a viable natural anti-inflammatory compound. Its application in diseases marked by chronic inflammation, such as arthritis and inflammatory bowel diseases, is thus promising (10-12). In the field of metabolic health, recent studies have explored the impact of lycopene on lipid metabolism and glucose homeostasis (13). Preliminary data indicate that lycopene may have favorable effects on lipid profiles and insulin sensitivity, suggesting a role in the prevention and management of metabolic syndromes, including diabetes. Moreover, lycopene's impact on cellular signaling pathways has been examined, particularly its regulatory effects on cell proliferation and apoptosis (14, 15). These findings imply potential applications in cancer prevention and treatment by targeting aberrant cellular processes. Given these multifaceted benefits, advancing the clinical application of lycopene is an endeavor that merits dedicated effort.
The therapeutic application of natural killer (NK) cells in the treatment of NSCLC has exhibited promising advancements (16). Recent studies have underscored the effectiveness of NK cell therapy in amplifying anti-tumor immune responses, which have been associated with tumor shrinkage and the enhancement of patient prognosis (17-19). One of the key advantages of NK cells is their innate ability to recognize and destroy cancer cells without the need for prior sensitization, positioning them as a highly adaptable treatment modality. Despite these promising developments, there are still challenges to be addressed. These include the limited in vivo persistence of NK cells and the tumor immuno-suppressive microenvironment that can hinder their efficacy. Current research efforts are directed toward developing strategies that can boost the durability of NK cells within the body and counteract the immunosuppressive forces within the tumor microenvironment. Such strategies are critical for augmenting the curative potential of NK cell-based therapies for NSCLC, thereby contributing to the evolution of cancer immunotherapy.
2. Objectives
Taken together, in this study, we aimed at demonstrating the synergistic effect of combining lycopene and NK cells in treating NSCLC and to explore the underlying mechanisms of such combination, which hope to offer a novel point of view in NSCLC treatments.
3. Methods
3.1. Cell Culture
Human cell lines HUVEC, BEAS-2B, NCI-H460, and A549 were purchased from Procell and cultured with 1640 medium supplemented with 10% FBS (20). Cells were cultured at 37℃ in a humidified atmosphere containing 5% CO2. Cells were sub-cultured every 2 days.
3.2. NK Cell Preparation
Primary NK cells were isolated from PBMC by using an NK Cell Isolation Kit (130-092-657; Miltenyi Biotec) following the manufacturer's introduction. NK cells were stimulated and expanded in an NK culture medium purchased from Miltenyi supplemented with human IL-2 (200 IU/mL). Lycopene (10 µM) was added on the first day of NK cell isolation. The culture medium was changed every 3 days. After a 7-day culture, NK cells were harvested for further detection.
3.3. Colony Formation
Cells were cultured in 6-well plates (1000 cells/well) overnight. Then, the cells were treated with lycopene and NK cells at a ratio of 1:1 for 4 hours. After treatment, NK cells were discarded and washed with PBS twice; 14 days later, cells were fixed and stained with 5% Giemsa solution. Cell colonies were photographed and counted with Image J software.
3.4. CCK-8 Assay
Different concentrations (0, 2.5, 5, and 10 μM) of lycopene were added and cultured with indicated cell lines for 24 hours. After incubation, the culture medium was discarded and washed with PBS twice. After washing, 10 μL CCK-8 reagent was added to each well followed by 1-hour incubation. Then, plates were measured at 450 nm with Nebula Absorbance Reader (Lonza). Control cell viability was taken as 100%.
3.5. FACS Detection
Live cell staining and CFSE staining were used to detect the live NK cells and NK cell proliferation, respectively. Shortly, apoptosis staining reagents were added and incubated for the indicated time before washing and after washing, harvest cells for flow cytometry detection. FACS data were further analyzed with Flow-Jo software.
3.6. IFN-γ and CD107α Secretion
NK cells were co-cultured with targeted NSCLC cells for 2 hours with or without lycopene supplement. After co-culture treatment, NK cells were harvested for Golgi-stop treatment and, then, incubated with indicated antibodies (anti-IFN-γ and anti-CD107α) following instructions (21). FACS data were further analyzed with Flow-Jo software.
3.7. ELISA
After co-culture as mentioned above, supernatant was harvested and centrifuged to discard cell debris. Elisa's detection was conducted following the manufacturer's instructions. IFN-γ and CD107α Elisa kits were purchased from Invitrogen (KHC4021, EH294RB).
3.8. RT-qPCR
All the indicated cells and tissue were dissociated with Trizol (Sigma-Aldrich; T9424) and RNA extraction was conducted according to the manufacturer’s instructions. The RNA products were reverse transcribed into cDNA, using PrimeScript™ RT-PCR Kit (TAKARA, Cat. # RR014A). RT-qPCR was performed as described and the primers used were listed in Table 1.
| Genes | Primers(5' - 3') |
|---|---|
| P60 | F: AATGGCTCGTCTGTAGTGC |
| R: TGCTCAATGATCTCCACATAGG | |
| TRAF6 | F: CCCAATTCCATGCACATTCAG |
| R: AGTCGGGTATAACGCTCAAAC | |
| P50 | F: GGATCTGGTTTCAGTGTCTCAG |
| R: CTGTTTAAGCGTGGATGCC | |
| c-Rel | F: AGAATTGTGGAAGTGTCAGAGG |
| R: AATGGCTACTTGACGGTGTAC | |
| Β-action (human) | F: TCGTGCGTGACATTAAGG |
| R: AAGGAAGGCTGGAAGAGT |
3.9. Statistical Analysis
In this study, “n” represents the number of biological repeats and the number of mice used for the experiment as indicated in figure legend. All the experiments were repeated independently at least 3 times and representative results were displayed. P-values for the comparison between groups were calculated, using either one way ANOVA or Student’s t-test. The level of significance was shown as: *P < 0.05, **P < 0.01, ***P < 0.001. GraphPad Prism 9 was used to analyze the data.
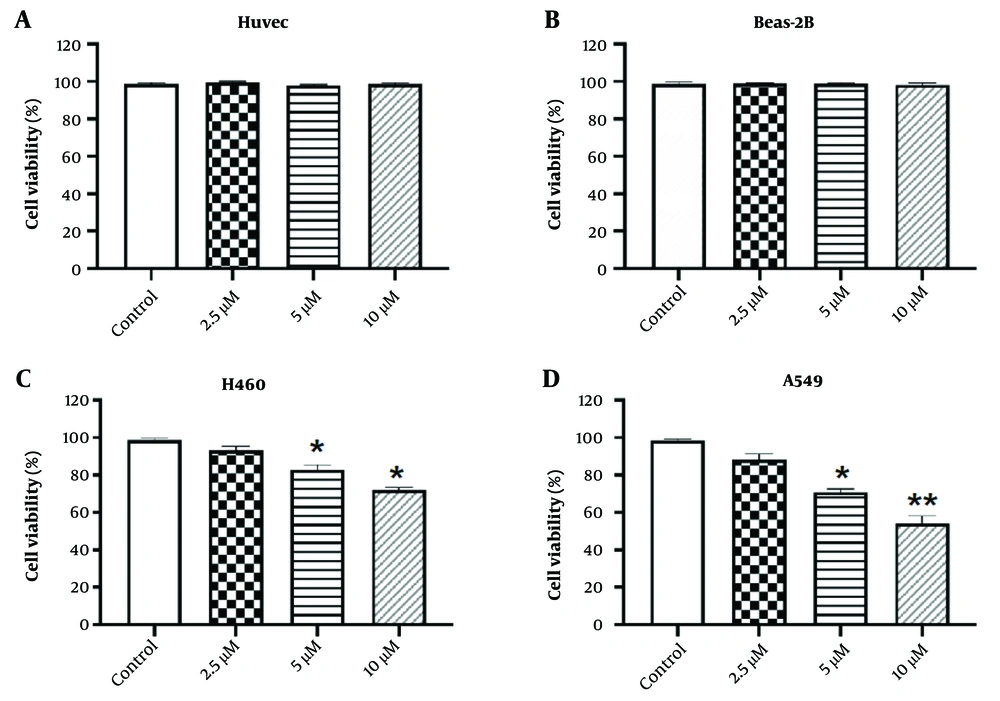
Lycopene has limited cytotoxicity towards NSCLC cells but not normal cells. A and B, normal human cell line HUVEC and Beas-2B were treated with different concentrations of lycopene as indicated. Cell viability was determined by CCK-8 assay. Control cell viability was taken as 100%; C and D), NSCLC cell lines H460 and A549 were treated with different concentrations of lycopene as indicated. Cell viability was determined by CCK-8 assay. Control cell viability was taken as 100%. n = 3, * P < 0.05 and ** P < 0.01 vs control.
4. Results
4.1. Lycopene has Limited Cytotoxicity on NSCLC Cells
To evaluate the biological impact of lycopene on NSCLC, it is critical to first determine its cytotoxic effects on normal human cells. As shown in Figure 1A-B, the CCK-8 assay indicated that lycopene at various concentrations (2.5, 5, and 10 µM) did not exhibit significant cytotoxicity towards normal human cell lines HUVEC and Beas-2B when compared to the control group. Subsequently, we further examined the effect of lycopene on NSCLC cells. Our CCK-8 assay results revealed that cell viability of NSCLC cells decreased in a dose-dependent manner (Figure 1C-D). Although there was a statistically significant difference in inhibition efficiency, the overall effect was moderate. These findings suggest that while lycopene has the potential to reduce the viability of NSCLC cells, its efficacy may be suboptimal and, thus, requires further enhancement. Consequently, it prompts an inquiry into whether lycopene could modulate the viability of NSCLC cells through an alternative mechanism.
4.2. Lycopene Promotes NK Cell Viability
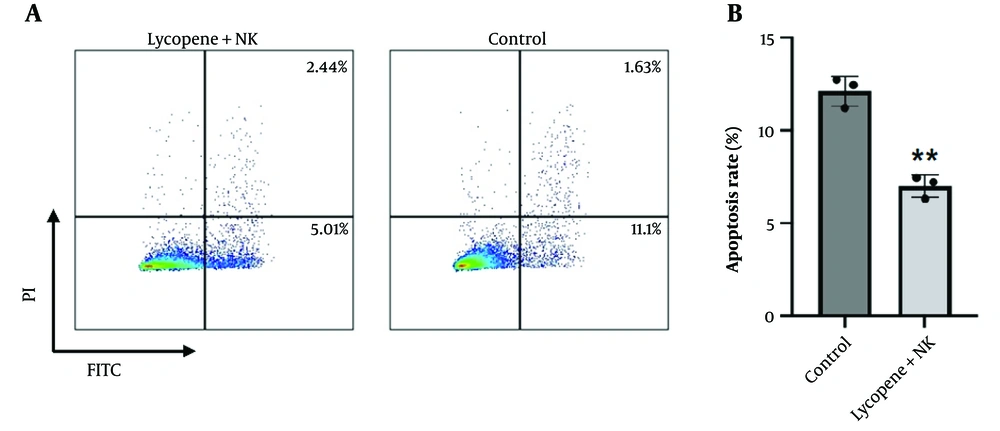
The pursuit of NK cell-based therapies is a promising frontier in precision and translational medicine, given the innate ability of NK cells to rapidly and robustly eliminate NSCLC cells. Nonetheless, a critical bottleneck in the clinical advancement of NK cell therapies is the observed decline in cell viability and proliferative capacity during in vitro passaging, which consequently diminishes their cytotoxic effectiveness. To address this challenge, we investigated the biological effects of lycopene on NK cells. Interestingly, our live cell staining assays revealed that lycopene supplementation enhances the viability of NK cells and is associated with a reduced percentage of apoptotic cells (Figure 2A-B). These findings suggest that lycopene may act as a supportive adjuvant for NK cells, promoting their in vitro viability and potentially augmenting their capacity to diminish NSCLC cells.
Lycopene promotes NK cell viability. A, NK cells were isolated from PBMC and cultured in vitro with or without lycopene supplement (10µM). After a 7-day culture, NK cells were further withdrawn for apoptosis staining; B, quantification analysis for apoptotic cells. n = 3, * P < 0.05 and ** P < 0.01 vs control.
4.3. Lycopene and NK Cells Exert Synergistic Effects on NSCLC Cells
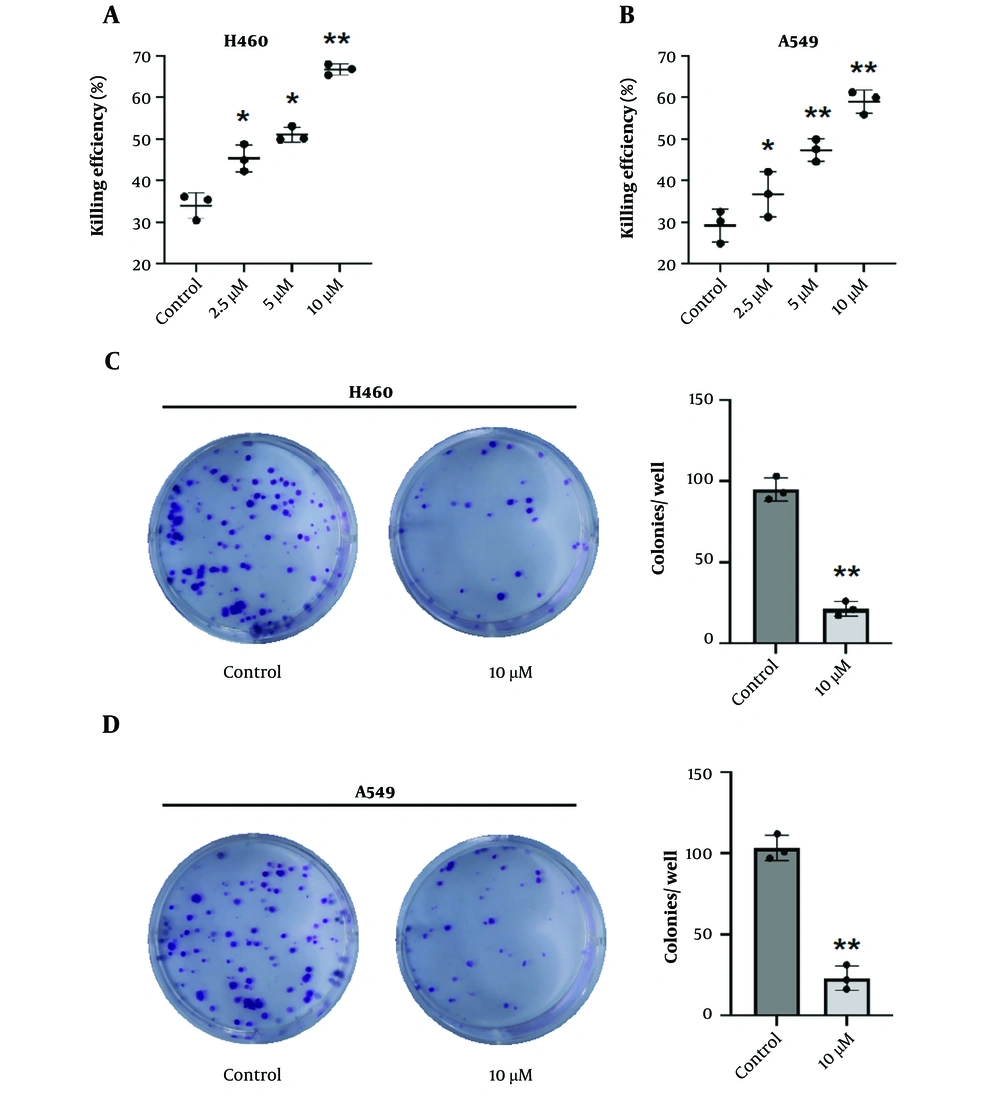
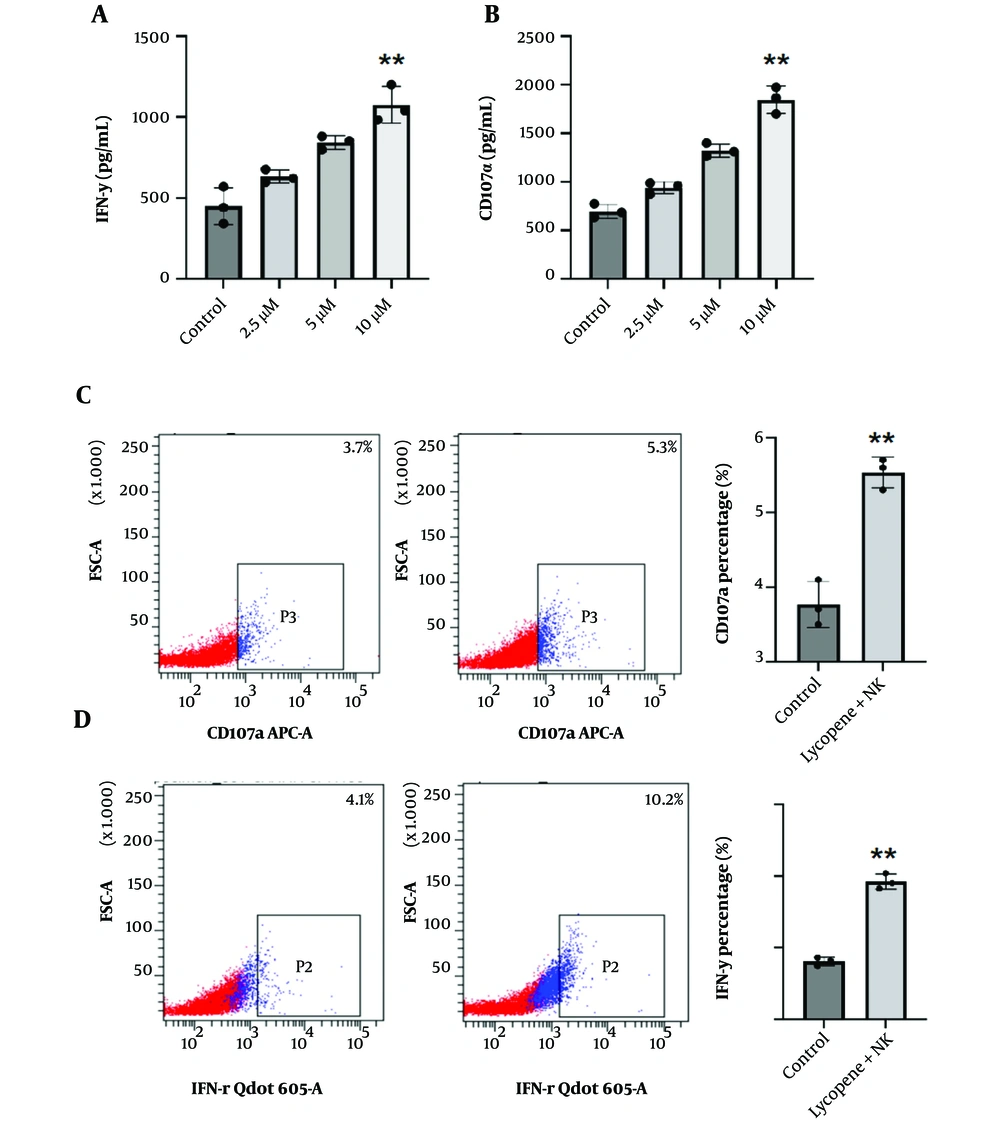
Consistent with the above evidence, we proceeded to examine the combined impact of NK cells and lycopene on the eradication of NSCLC. NK cells were harvested from cultures with and without a 7-day lycopene treatment as mentioned above. As we expected, the cytotoxic efficacy against NSCLC was significantly improved when lycopene was included in the treatment, compared to the use of NK cells alone (Figure 3A-B). In addition, the colony formation assay also indicated the lycopene supplement significantly inhibited NSCLC colony formation comparing NK cells alone (Figure 3C-D). Moreover, we observed that after lycopene supplementation, there was a significant increase in the secretion of IFN-γ and CD107α, which are critical markers of NK cell function (Figure 4A-D). These results suggest that lycopene can enhance the cytotoxicity of NK cells, indicating a synergistic effect when both NK cells and lycopene are combined. This synergy points towards a promising avenue for further investigation in the context of NSCLC translational therapy.
Lycopene enhances NK cell cytotoxicity towards NSCLC. A, H460 and B, A549 NSCLC cells were co-incubated with NK cells at a ratio of 1:1 with or without lycopene supplement as indicated concentrations. 4 hours later, an LDH-releasing assay was conducted to calculate the killing efficiency of NK cells. n = 3. C, H460 and D, A549 NSCLC cell co-culture with indicated NK cells was performed and the supernatant was discarded following supplement fresh growth medium. 10 days later, colony formation was photographed with a representative graph shown here. Quantification analysis was performed with Image-J. n = 3, * P < 0.05 and ** P < 0.01 vs control.
Lycopene promotes IFN-γ and CD107α secretion of NK cells. A-B, to assess NK cell functions, co-culture with target NSCLC cells was conducted and 2 hours later, the supernatant was collected for further Elisa detection. A, IFN-γ; and B, CD107α concentrations were determined. n = 3, C-D, representative flow cytometry detection of C, IFN-γ; and D, CD107α of indicated NK cells staining. Quantification analysis was performed. n = 3, * P < 0.05 and ** P < 0.01 vs control.
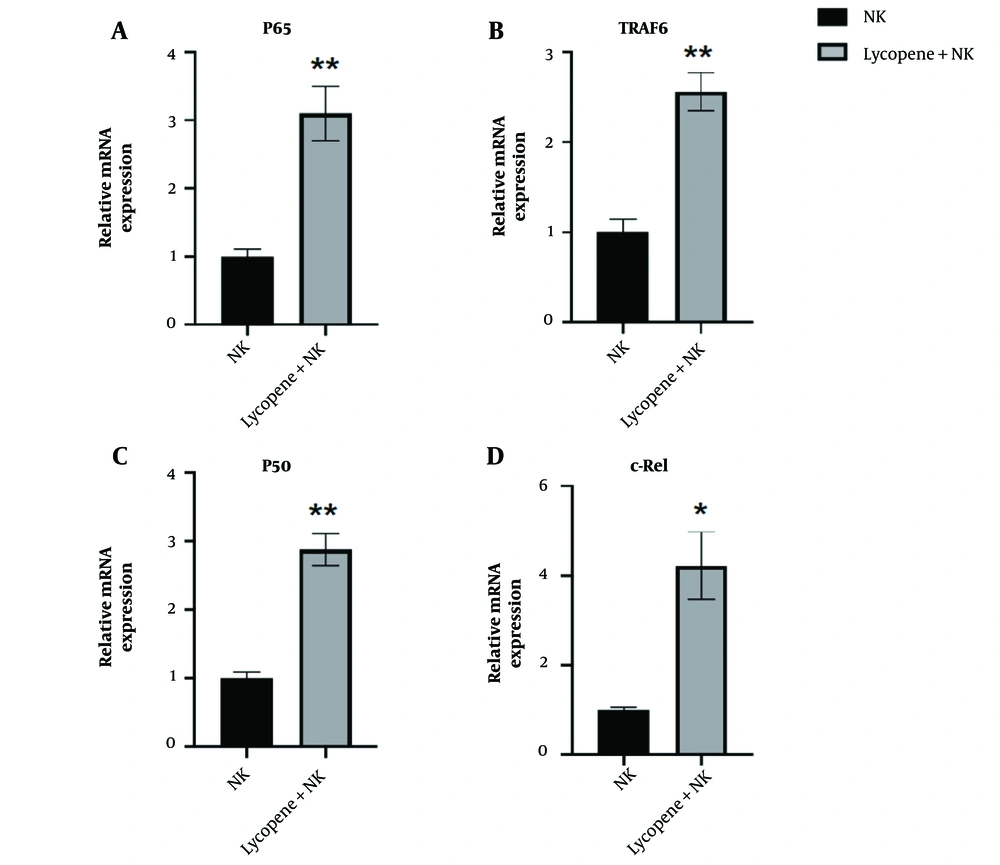
4.4. Lycopene Enhances NK Cell Function by Activating NF-κB Signaling Pathway
Next, it is imperative to elucidate the underlying mechanism, by which lycopene promotes NK cell functions. According to the literature review, the NF-κB signaling pathway is known to play a vital role in regulating NK cell activation and functions. Consistent with this idea, we measured the RNA expression levels involved in the NF-κB signaling pathway. The results aligned with our expectations. The RNA expression of P65, TRAF6, c-Rel, and P50 were all upregulated after lycopene supplementation, which indicated the activation of NF-κB activation (Figure 5A-D). From our results, we can conclude that lycopene's promotion of NK cell function is likely associated with the activation of the NF-κB signaling pathway, providing insights for subsequent translational clinical research.
5. Discussion
The findings presented in our study provide valuable insights into the biological effects of lycopene on NSCLC cells and NK cells, opening avenues for potential therapeutic strategies. Notably, our results demonstrate that lycopene exhibits limited cytotoxicity in normal human cells, suggesting its potential safety for further therapeutic exploration. However, its efficacy against NSCLC cells is moderate, indicating room for improvement. One of the promising aspects highlighted in our study is the positive impact of lycopene on NK cell viability and proliferation. Interestingly, we speculate that the differential role of lycopene in treating NSCLC and NK cells may primarily depend on the distinct genomic stability inherent in NSCLC and NK cells. For example, P53 is frequently mutated in NSCLC, a target that lycopene might engage to exert an anti-proliferative effect (22). Given the crucial role of NK cells in the rapid and aggressive elimination of NSCLC cells, it is essential to enhance their viability and proliferation to develop effective cell-based therapies. Our live cell staining assay reveals a dosage-dependent increase in NK cell viability and proliferation upon lycopene supplementation, underscoring its potential as a supportive adjuvant for NK cell-based therapies.
Building on this, the observed synergistic effect of combining NK cells with lycopene further underscores its potential to enhance cytotoxicity against NSCLC cells. The significant increase in killing efficiency and the inhibition of colony formation following lycopene supplementation provides a strong rationale for exploring this combination in translational therapy. Moreover, upregulation of IFN-γ and CD107α secretion following lycopene supplementation indicates an improved NK cell function, reinforcing the potential synergistic benefits in NSCLC treatment. To elucidate the underlying mechanisms, our study investigated the NF-κB signaling pathway, which is known for its regulatory role in NK cell activation and functions. The upregulation of key RNA expressions involved in this pathway following lycopene supplementation suggests its activation and provides a potential mechanistic link to the observed enhancement in NK cell function. This insight into the molecular pathways involved paves the way for targeted interventions in future clinical developments.
However, it is essential to acknowledge the limitations of our current study. The moderate efficacy of lycopene against NSCLC cells suggests the need for further optimization of treatment regimens or exploration of combinatory approaches. Additionally, while our results provide a foundation for comprehending the role of lycopene in NK cell function, detailed mechanistic studies are necessary to fully elucidate the complete signaling cascades involved. In addition, an in vivo assessment might be imperative to demonstrate further the synergistic effect of combining lycopene and NK cell therapy.
In conclusion, our study presents compelling evidence for the potential of lycopene as an adjuvant in NK cell-based therapies for NSCLC. The observed synergistic effects and activation of the NF-κB signaling pathway pave the way for future translational research. Addressing the limitations and further optimizing treatment strategies will be crucial for maximizing the therapeutic potential of lycopene in precision medicine for NSCLC.