1. Background
Renal cell carcinoma (RCC) is among the most common cancers globally, accounting for 2% to 3% of all cancer cases (1). It ranks as the 9th most prevalent cancer in men and the 14th in women (2). Over recent decades, the incidence of RCC has been rising, partly due to increased cancer detection rates, especially in developing countries (3). Several risk factors have been linked to RCC, including male gender, smoking, obesity, and hypertension (1, 3, 4). Prognostic factors for RCC are typically categorized into four groups: Anatomic, histologic, clinical, and molecular factors. Furthermore, treatment interventions can significantly impact patients’ long-term survival.
Numerous studies have sought to evaluate and clarify the effects of these variables on RCC prognosis. Some factors, such as disease stage and histologic subtype, have well-established roles in predicting outcomes (5, 6). However, the roles of other factors, including specific histologic subtypes, gender, and type of surgery, remain uncertain, with conflicting findings across studies. Additionally, most studies and predictive models have been developed in Western countries, and there is a lack of research on survival factors in RCC patients in the Middle East.
2. Objectives
Therefore, this study aims to evaluate factors associated with RCC outcomes in patients diagnosed at the primary oncologic centers and hospitals in Mashhad, Iran.
3. Methods
3.1. Patient Population and Study Variables
In this historical cohort study, all patients with a histological diagnosis of RCC who were referred to the oncological centers of Mashhad University of Medical Sciences—namely, Omid and Imam Reza Hospital and Reza Radiation Oncology Center—from 2001 to 2016 were included. Data were extracted from patients’ hospital records, and then each patient was contacted via the phone numbers provided in their records to obtain information regarding their current status, survival, and disease-free period. Informed consent was obtained from all participants. This study was conducted under the oversight and approval of the Mashhad University of Medical Sciences ethics committee (code: 1396.269), and no additional interventions were performed on the participants.
Study variables included sociodemographic characteristics such as age at diagnosis, sex, and body mass index, as well as clinical factors, including presenting symptoms, cancer stage and grade, type of surgical intervention, chemotherapy regimen, laboratory data (e.g., hemoglobin levels), and histopathologic classification of tumors. The primary outcome variable was disease-free survival (from diagnosis to the occurrence of local recurrence or distant metastasis), and the secondary outcome variable was overall survival (from diagnosis to death from any cause or the patient’s last follow-up visit).
3.2. Statistical Analysis
Data were analyzed using standard descriptive statistics and frequency tables. Patient survival rates were estimated with the Kaplan-Meier method, and the Cox proportional-hazards model was employed to examine the association between survival time and predictor variables. Data analysis was performed with SPSS software version 23, and a significance level of P < 0.05 was applied throughout the investigation.
4. Results
A total of 230 patients were enrolled in the study, with a mean age of 56.78 years. Of these, 142 (61.7%) were male. The predominant histologic subtype of RCC was clear cell carcinoma (70.5%). The most prevalent tumor grade observed was grade III (36.9%), and the most common disease stage was stage IV (31%). Pain emerged as the most frequently reported symptom among patients. Additional demographic data, clinicopathologic characteristics, treatment details, and outcomes are presented in Table 1.
| Variables | Total = 230 |
|---|---|
| Gender | |
| Male | 142 (61.7) |
| Female | 88 (38.3) |
| BMI | |
| < 18.5 | 18 (10.2) |
| 18.5 - 25 | 74 (41.8) |
| 25 - 30 | 56 (31.6) |
| > 30 | 29 (16.4) |
| Hemoglobin at diagnosis | |
| < 10 | 39 (20.5) |
| > 10 | 151 (79.5) |
| Symptom | |
| Pain | 119 (64.7) |
| Hematuria | 60 (32.6) |
| Flunk mass | 37 (20.1) |
| Weight loss | 43 (18.7) |
| Others | 27 (14.6) |
| Pathologic subtype | |
| Clear cell | 162 (70.5) |
| Papillary Type I | 11 (4.8) |
| Papillary Type II | 10 (4.3) |
| Chromophobe | 4 (1.7) |
| Sarcomatoid | 22 (9.5) |
| Mixed cell | 1 (4.3) |
| Rhabdoid | 2 (8.6) |
| Spindle cell | 1 (4.3) |
| Missing | 17 (7.3) |
| Tumor grade | |
| I | 22 (9.5) |
| II | 68 (29.5) |
| III | 85 (36.9) |
| Missing | 55 (23.9) |
| Stage | |
| I | 45 (19.5) |
| II | 37 (16.1) |
| III | 65 (28.2) |
| IV | 71 (31) |
| Missing | 12 (5.2) |
| Surgery type | |
| Radical nephrectomy | 197 (86) |
| Partial nephrectomy | 16 (7) |
| None | 17 (7) |
| Systemic therapy | |
| Sunitinib | 23/56 (41) |
| Other | 33/56 (59) |
| Recurrence type | |
| Local | 10/129 (7.8) |
| Distant | 116/129 (89.9) |
| Both | 3/129 (2.3) |
| Metastatic site | |
| Bone | 52/119 (43.7) |
| Lung | 28/119 (23.5) |
| Liver | 22/119 (18.5) |
| Others | 17/119 (14.3) |
Abbreviation: BMI, Body Mass Index.
a Values are expressed as No. (%).
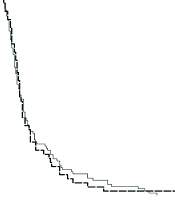
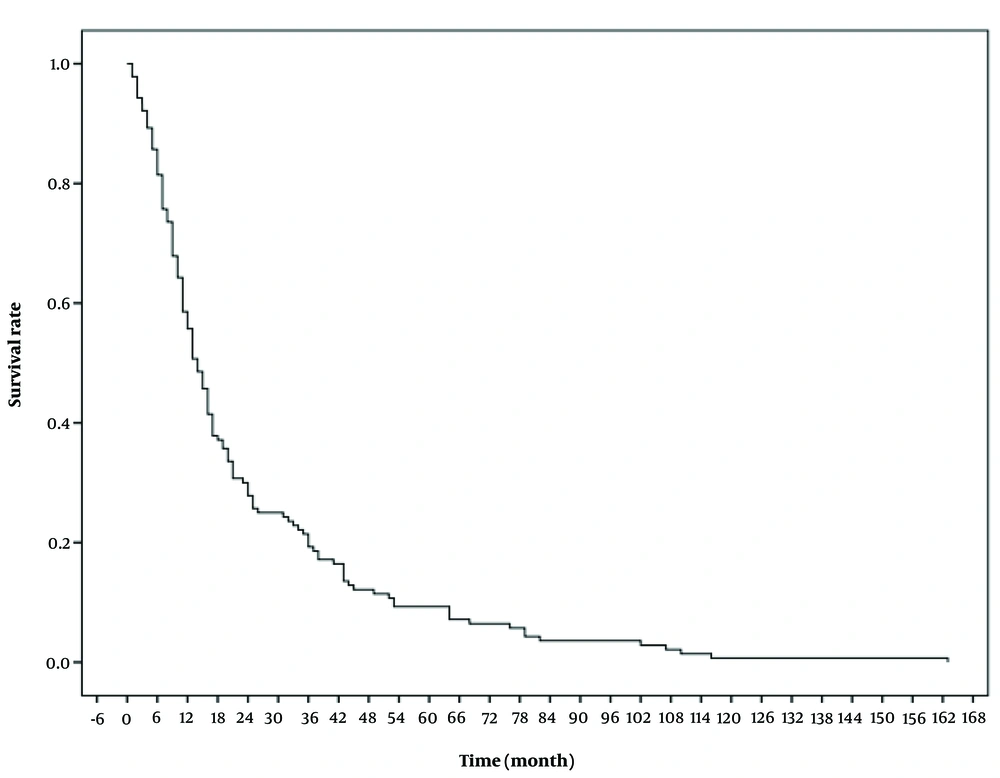
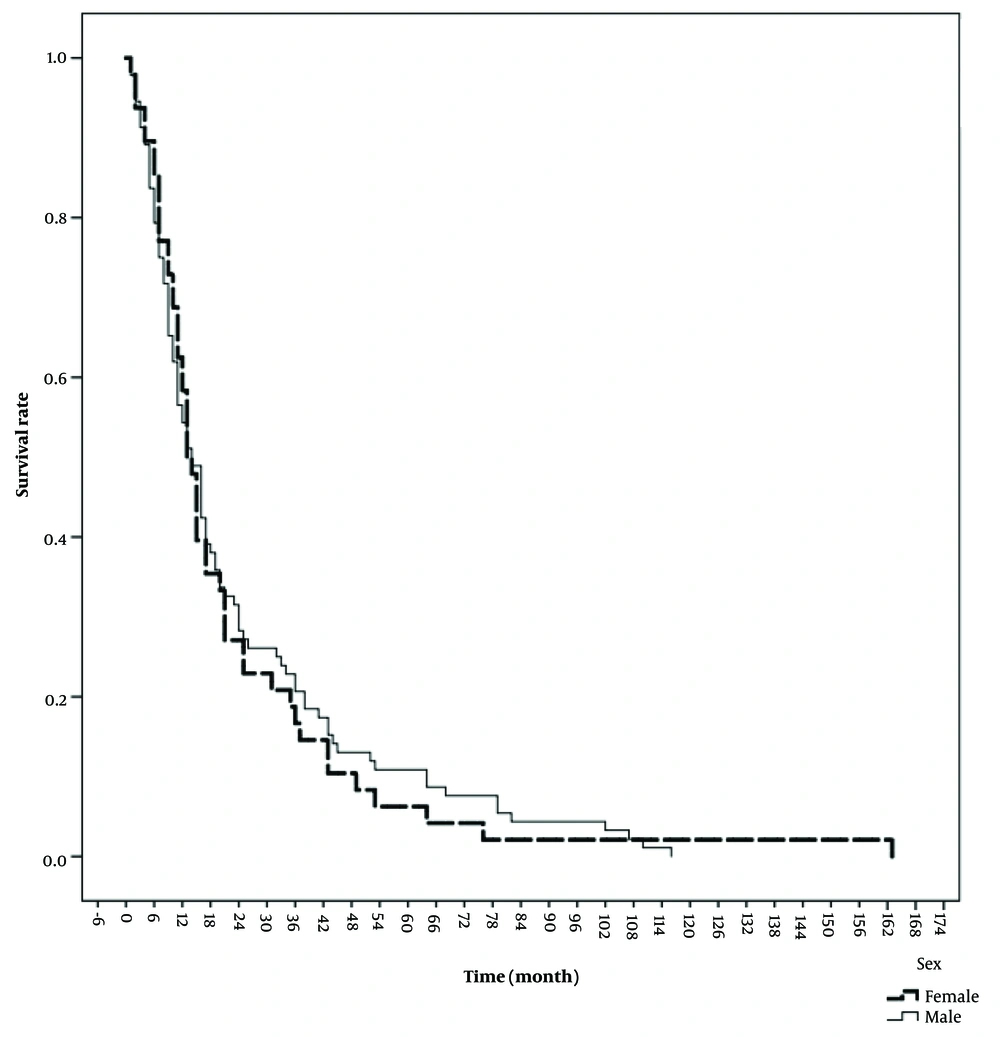
The median follow-up period for the patients was 10.50 months (IQR = 3.00 - 30.50). During this study, a total of 140 patients died. The mean overall survival (OS) was 24 ± 2 months, and the mean disease-free survival (DFS) was 25 ± 2 months. Table 2 presents the 1, 3, 5, and 10-year overall survival rates, recorded as 74.8%, 52.2%, 44.8%, and 39.6%, respectively. Figure 1 illustrates the patients' OS, while Figure 2 provides a comparison of OS by gender. The median OS for women was 13 months (IQR = 10.7 - 15.2), and for men, it was 14 months (IQR = 10.4 - 17.6) (P = 0.74). The median DFS was 8 months (IQR = 1 - 28.2) in men and 7 months (IQR = 1 - 24.2) in women (P = 0.53).
| Overall Survival Rate | No. (%) | P-Value |
|---|---|---|
| One-year OS | 172 (74.8) | 0.01 |
| 3-year OS | 120 (52.2) | |
| 5- year OS | 103 (44.8) | |
| 10-year OS | 91 (39.6) |
The results of the univariate analysis showed that the key factors significantly affecting patient survival were disease stage and histologic subtype. Both OS and DFS declined substantially as the disease stage advanced, with Stage IV having a markedly negative impact on patient outcomes (P < 0.001). In terms of histologic subtype, papillary type I showed the highest survival at 41 months, followed by clear cell, sarcomatoid, and papillary type II, with survival times of 15, 12, and 6 months, respectively (P = 0.004).
Patients were categorized into four groups based on BMI, ranging from underweight to obese. The univariate analysis results indicated that OS was significantly lower in underweight patients (BMI < 18.5) compared to others (P = 0.04). However, DFS did not differ significantly among the BMI groups (P = 0.53). For serum hemoglobin levels, patients with hemoglobin levels below 10 had significantly lower survival than those with levels above 10 (P = 0.03). The survival of patients who underwent radical nephrectomy was slightly better than those who had partial nephrectomy, though not significantly (OS: 19 vs. 15 months, P = 0.5; DFS: 21 vs. 9 months, P = 0.27).
In comparing drug regimens, the analysis showed that the median survival for patients treated with sunitinib was 8 months (IQR = 5.0 - 16.7), while the median OS for those on other medications was 13 months (IQR = 8 - 24) (P = 0.14).
According to the Cox proportional-hazards model (P = 0.004), among the variables of sex, hemoglobin level, BMI, disease stage, grade, and histologic subtype, grade and histologic subtype were significant predictors of OS (P < 0.05) (Table 3).
| Variables | Median OS (month) | Odd Ratio | P-Value |
|---|---|---|---|
| Histologic subtype | |||
| Clear cell | 15 (12.4 - 17.5) | Reference | 0.02 |
| Papillary type 1 | 41 (20.4 - 61.5) | 0.32 | 0.09 |
| Papillary type 2 | 6 (2.4 - 9.6) | 2.39 | 0.38 |
| Sarcomatoid | 12 (7.9 - 16.1) | 0.37 | 0.26 |
| Other types | 13 (0 - 31.9) | - | - |
| Primary stage | |||
| 1 | 36 (21.9 - 50.1) | Reference | 0.004 |
| 2 | 21 (0 - 48.5) | 0.12 | 0.007 |
| 3 | 14 (12.1 - 15.8) | 0.14 | 0.001 |
| 4 | 10 (7.6 - 12.3) | 0.48 | 0.06 |
| Grade | |||
| 1 | 32 (13 - 88) | Reference | |
| 2 | 12 (6.5 - 28.5) | 2.98 | 0.43 |
| 3 | 11 (7 - 24) | 3.17 | 0.33 |
| Gender | 0.87 | ||
| Female | 13 (10.7 - 15.2) | Reference | |
| Male | 14 (10.4 - 17.6) | 1.06 | |
| BMI | |||
| < 18.5 | 9 (2.1 - 16) | Reference | 0.48 |
| 18.5 - 25 | 13 (9.5 - 16.5) | 1.39 | 0.58 |
| 25 - 30 | 10 (4.6 - 15.4) | 1.93 | 0.18 |
| > 30 | 15 (6.7 - 23.2) | 1.94 | 0.16 |
| Hgb | 0.41 | ||
| > 10 | 11 (8.03 - 13.97) | Reference | |
| < 10 | 15 (11.71 - 18.28) | 1.33 |
5. Discussion
The incidence rate of RCC has been increasing globally, a trend that cannot be solely attributed to the growing use of imaging studies (1, 2). Numerous studies have identified various factors associated with the outcomes of RCC patients, including disease stage, histologic subtype, type of surgery, medical treatment, gender, BMI, and anemia levels. The prognostic value of histologic subtype is well-documented in the literature, though prior studies report mixed results regarding the impact of specific subtypes on RCC prognosis. In our study, patients with the papillary type I subtype had the best survival rates, while those with papillary type II showed the worst outcomes. Patard et al., in their study of 4,063 patients with clear cell, papillary, and chromophobe pathology, found that histologic subtype was not significantly associated with survival (7). Conversely, Leibovich et al., with a population of 3,062 RCC patients, and Teloken et al., with 1,863 patients, reported that survival was significantly worse in patients with clear cell pathology compared to other subtypes (5, 8).
Consistent with our findings, previous studies indicate that disease stage is significantly associated with patient outcomes, with Stage IV disease having a markedly negative effect on prognosis (7, 9, 10).
Regarding the association between surgical type and patient outcomes, our study found that survival rates for patients who underwent radical nephrectomy were not significantly better than those for patients who had partial nephrectomy. It is important to note that patients undergoing partial and radical nephrectomies may differ considerably in terms of disease stage, comorbidities, and other factors. The absence of a significant survival difference may be due to the secondary complications of end-stage renal disease, which occur more frequently after radical nephrectomy compared to partial nephrectomy. This finding aligns with prior studies showing no significant association between overall survival and the type of surgical procedure in RCC patients (11). However, a recent study by Ristau et al. reported that partial nephrectomy was associated with improved overall survival compared to radical nephrectomy in patients with T1A RCC (12).
In this study, we found that the serum hemoglobin level of patients had a significant impact on survival. This finding aligns with previous reports on RCC patient outcomes across different countries. In a large multicenter study, Heng et al. reported that anemia was independently associated with shorter survival in metastatic RCC cases receiving vascular endothelial growth factor-targeted treatment (13). Similarly, Peng et al. emphasized the prognostic importance of combining preoperative hemoglobin and albumin levels with lymphocyte and platelet counts (HALP) in predicting outcomes for RCC patients (14). Additional studies suggest that hemoglobin levels may also serve as an indicator of tyrosine kinase inhibitor efficacy (e.g., sunitinib), correlating with improved outcomes in metastatic RCC patients (15, 16). The presence of anemia at the time of RCC diagnosis is often associated with a more advanced cancer stage (17). Cancer-related anemia may arise from factors such as blood loss, nutritional deficiencies like cobalamin deficiency, or dysfunctional inflammatory responses and mechanisms (14).
The impact of gender on patient outcomes has been a point of debate in previous studies. While some studies suggest that being male is associated with a less favorable outcome, other studies, which adjusted for histologic grading and the presence of metastasis, found no association between gender and disease outcome (18-20). In our study, we also found no significant association between gender and oncological outcomes.
Another poor prognostic factor, which aligns with findings from previous studies, was being underweight. Numerous studies highlight the significance of BMI on cancer-specific survival in RCC patients (21-25). A meta-analysis by Bagheri et al. reported that while survival improves for RCC patients with a BMI within the normal range, outcomes may begin to worsen as BMI reaches the overweight category (BMI > 25) (23). Additionally, a study by Bookman-May et al. found that preoperative weight loss has a more prominent impact on prognosis than BMI itself (24). This difference could be attributed to varying levels of growth factors or immune responses between underweight and normal-weight individuals; however, the exact pathophysiological mechanisms underlying this phenomenon remain to be clarified (24).
5.1. Limitations and Strengths
Few studies have examined RCC survival in Iran, and this study represents the largest investigation to date into the factors affecting RCC patient survival in the region. The variety of factors evaluated, along with the number of patients enrolled and the follow-up period, contribute to potentially reliable and valid findings. However, this study is not without limitations, including a relatively small sample size and a focus on short-term outcomes. The study would have been strengthened by a multicenter design. Additionally, there may be other factors influencing patient survival that were not addressed in this study.
5.2. Conclusions
In this study conducted in Iran, we found that the 1-, 3-, 5-, and 10-year overall survival rates were 74.8%, 52.2%, 44.8%, and 39.6%, respectively, with mean overall survival and disease-free survival rates of approximately 24 and 25 months, respectively. The factors that significantly impacted RCC patient survival were histologic subtype, disease stage, anemia, and being underweight. However, our study did not reveal a statistically significant association between gender, type of surgery, or medical therapy regimens and patient survival.