1. Introduction
Wilms tumor (WT) or nephroblastoma is one of the most common childhood malignancies, which primarily involves renal tissue (1). The tumor originates from nephrogenic blastoma and imitates the histology of developing kidneys (2). Extrarenal WT is rare and occurs in 0.5 to 1% of WTs, mostly in children (3, 4). The most common extrarenal locations of WT include the retroperitoneum, inguinal area, lumbosacral and pelvic cavity, mediastinum, chest wall, spermatic cord/paratesticular region, and female genital organs (3).
Microscopically, classic WT is composed of three components such as blastemal, mesenchymal, and epithelial. Blastemal component consists of small to medium-sized undifferentiated cells with high mitotic activity. Epithelial components may consist of well-differentiated glandular, tubular or glomerular structures or poorly differentiated rosette-like structures. The stromal component includes spindle cells with or without heterologous elements (5).
Carcinosarcoma or malignant mixed mullerian tumor is a rare biphasic aggressive tumor of the uterine corpus. Carcinosarcoma mostly occurs in post-menopausal women. Microscopically, the tumor is composed of high-grade epithelial carcinoma and high-grade sarcoma. The sarcomatous component is derived from carcinoma cells as a result of epithelial-mesenchymal trans-differentiation. Heterologous rhabdomyosarcoma, chondrosarcoma or osteosarcoma components may also be present (6).
Herein, we reported a 63-year-old woman with advanced staged uterine cancer with epithelial and stromal components which was primarily misdiagnosed as carcinosarcoma. After the review of the slides by expert Gynecopathologists, the diagnosis was changed to uterine WT. Adult uterine WT is extremely rare with only 10 reported cases in English Literature.
Alpha fetoprotein (AFP) is an oncofetal protein of typically hepatoid and germ cell tumors, especially yolk sac tumor (7). Increased serum level of AFP in WT is not usual. There are few reported cases in this regard (7-10). Elevated serum AFP is the other interesting aspect of this case report.
2. Case Presentation
A 63-year-old menopausal woman, G5 L4 D1 presented with continuous mild lower abdominal pain for one month before her admission to the hospital. She had diabetes mellitus, hypertension, and hyperlipidemia and was under medical treatment. Her sister has been treated for thyroid cancer. On physical examination, vital signs were stable and uterus was larger than normal size. No other abnormal finding was detected. Abdominal and pelvic sonography and MRI showed 19 × 10 × 9 cm pelvic mass with uterine origin, suggestive of sarcoma. The cervix and adnexae were free from the tumor. Upper GI endoscopy, colonoscopy, and lung CT scan were normal. No primary liver mass or metastasis was identified. Evaluation of serum tumor markers revealed LDH: 784 IU/mL, CA 19-9: 503 IU/mL, CA 125: 302 IU/mL, ROMA: 94 IU/mL, AFP: 30 IU/mL. A biopsy of the uterine mass revealed necrotic material and a spindle tumor suggestive of sarcoma.
The patient underwent a total abdominal hysterectomy, bilateral salpingo-oophorectomy, and omentectomy. On macroscopic examination, the uterus was large measuring 15 × 11 × 10 cm and deformed. Endometrial cavity was irregular containing tan-yellowish fragile projections that filled the entire endometrial cavity and extended to the myometrium. The serosal surface was ruptured and involved by a tumor. The cervix, ovaries, and fallopian tubes were unremarkable. An omental mass measuring 3.5 cm in greatest dimension was also seen. According to the presence of mixed epithelial-mesenchymal structures and pathologic diagnosis of carcinosarcoma (pathologic stage IV), adjuvant chemotherapy with carboplatin, paclitaxel regimen, and external beam radiotherapy were prescribed. In 6 months follow up after treatment, no residual tumor was detected on imaging studies. Three months after the end of treatment, the patient presented with sub-hepatic recurrent mass and abdominal lymphadenopathies.
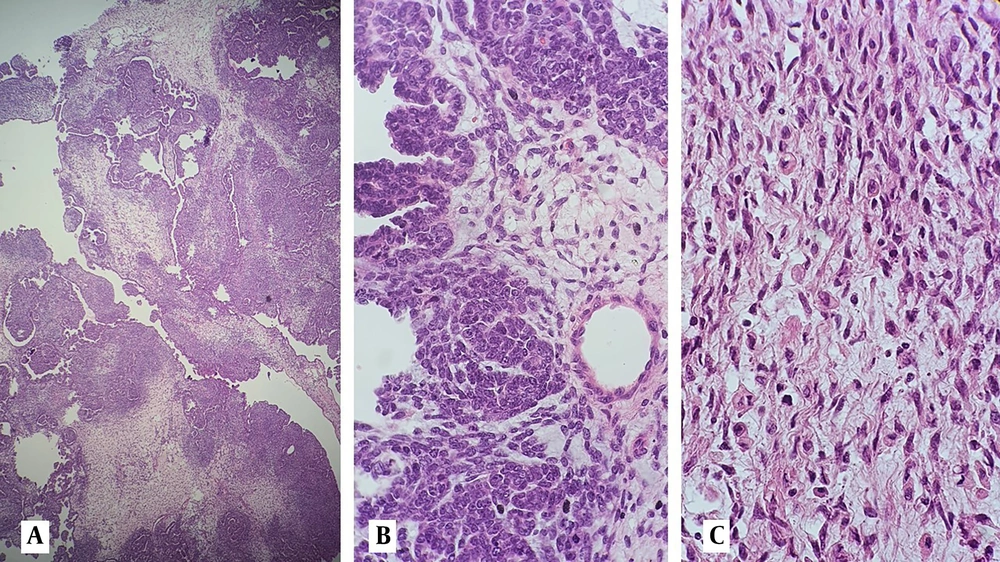
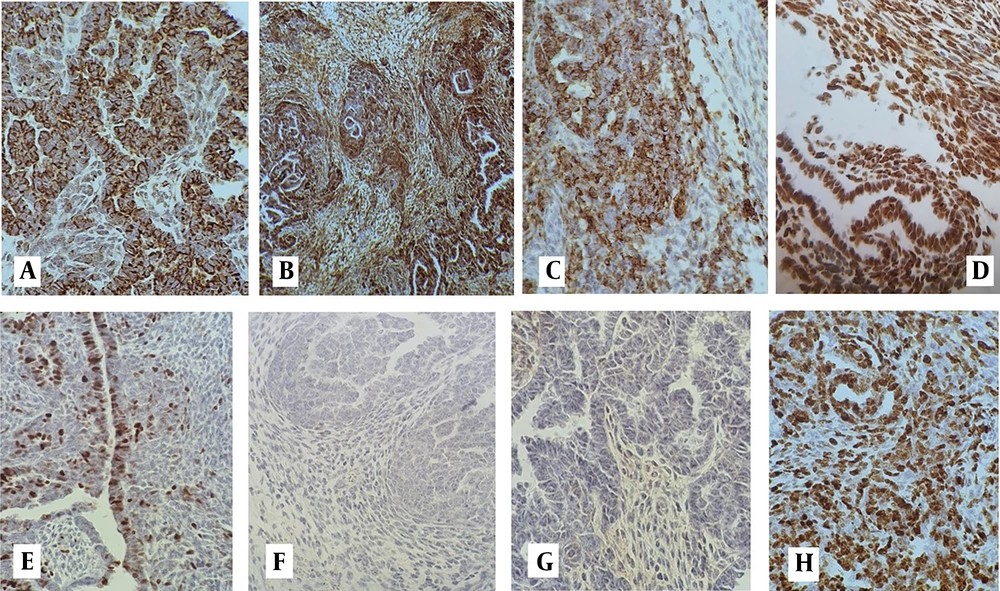
On review of pathology slides in another center (Yas Women's Hospital), a microscopic examination showed a triphasic tumor composed of epithelial, mesenchymal, and blastemal components. The epithelial component consisted of papillary structures, primitive tubules, and glomeruloid structures compatible with WT. Heterologous rhabdomyoblastic differentiation was also seen in the mesenchymal component (Figures 1 and 2). A complementary immunohistochemical (IHC) study showed a positive reaction for WT1, CKAE1 /AE3, EMA, Glypican-3, desmin, and CD56 in all components of the tumor, while P53 expression was normal. Tumor was negative in staining for GATA3, ER, PR, and CD10 (Figure 3A – H and Table 1). The patient received a second line of chemotherapy for WT. Unfortunately, she died 3 months after initiation of the treatment.
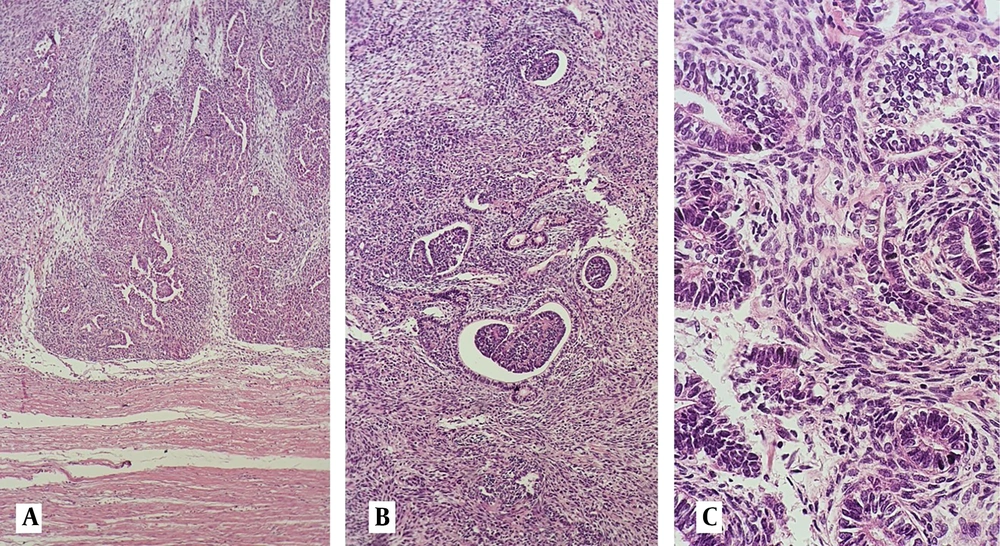
Microscopic examination of hematoxilin and eosin-stained sections shows; A, infiltration of myometrial tissue with a triphassic tumor composed of epithelial, blastemal, and mesenchymal structures (40X); B, glomeruloid structures and primitive tubules surrounded by spindle cells (100X); C, epithelial cells of the tubular structures and spindle cells showing mild nuclear atypia at high power examination (400X).
A, On low-power examination, leaf-like appearance and peri glandular cuffing mimic mullerian adenosarcoma (40X); B, Papillary structures are short, small, and non-branching, lined by epithelial cells with minimal atypia (100X); C, Heterologous rhabdomyoblastic differentiation in the mesenchymal component is seen (400X).
| IHC Marker | Reactivity in Tumor Cells of Epithelial Component | Reactivity in Tumor Cells of Mesenchymal Component | Reactivity in Tumor Cells of Blastemal Component |
|---|---|---|---|
| CK AE1/AE3 | + | +/- | + |
| EMA | + | +/- | + |
| WT1 | + | +/- | + |
| Glypican-3 | + | + | + |
| Desmin | +/- | + | +/- |
| CD56 | + | + | + |
| P53 | Normal | Normal | Normal |
| GATA3 | - | - | - |
| ER | - | - | - |
| PR | - | - | - |
| CD10 | - | - | - |
3. Discussion
The first case of extrarenal WT (in the mediastinum) was reported in 1961 by Moyson et al. (3, 11). The first case of uterine WT was reported by Bittencourt et al. in 1981 (12). Till now, only 10 cases of uterine corpus WT have been reported (Table 2) (13-20). Age of the patients ranged between 14 and 77 years. The most common symptom was abnormal uterine bleeding followed by abdominal pain and abdominal mass. About half of the reported cases revealed extrauterine spread at the time of diagnosis. Except for one case, which was inoperable at the time of diagnosis, the others were treated by TAH, BSO surgery, and adjuvant treatment (radiation, chemotherapy, or both). The molecular alteration was evaluated by Alessandrini et al. in the most recent extrarenal WT case (13). Molecular analysis revealed no microsatellite instability and unaltered tumor mutational burden. Copy number variations in the ERBB2, FGFR23, FGF6, FGFR2, and RPS6KB1 genes were reported.
| First Author | Year of Publication | Age | Clinical Presentation | Extrauterine Organ Involvement | Treatment | Outcome | Follow-up Time |
|---|---|---|---|---|---|---|---|
| Bittencourt (12) | 1981 | 14 | Abdominal pain and mass | Fallopian tube and posterior vagina | TAH, BSO, radiation, chemotherapy | alive | 5 years |
| Comerci (16) | 1993 | 22 | AUB | No | TAH | alive | 2 years |
| Jiskoot (17) | 1999 | 77 | Polypoid mass, AUB | Positive abdominal washing cytology | TAH, BSO, radiation | alive | 4 months |
| Muc (19) | 2001 | 42 | AUB, protruded mass | Transmural uterine extension | TAH, BSO (subtotal), radiation | Recurrence; dead | 6 months later; 1 year after |
| McAlpine (14) | 2005 | 44 | AUB | Parametrium, sacrouterine ligament | TAH, BSO, complete staging, chemotherapy | alive | 1 year |
| Leblebici (18) | 2009 | 16 | Abdominal pain, AUB, weight loss | Chemotherapy (inoperable) | died | A few days after chemo | |
| Garsia Galris (15) | 2009 | 62 | AUB | No | TAH, BSO, radiation, chemotherapy | alive | 14 months |
| Cao et al. (21) | 2017 | 60 | AUB | No | TAH, BSO, chemotherapy | alive | 18 months |
| Pinto (20) | 2018 | 33 | AUB, pelvic pain | No | Radical hysterectomy, BSO, omentectomy | Not applicable (NA) | NA |
| Alessandrini (13) | 2023 | 59 | Abdominal pain | No | TAH, BSO, omentectomy, appendectomyradiation, chemotherapy | alive | 6 months |
| Present case | 2024 | 63 | Abdominal and pelvic pain | Transmural uterine serosa and omentum | TAH, BSO, omentectomy, radiation, chemotherapy | Recurrence; Dead | 3 months |
Abbreviation: AUB, Abnormal uterine bleeding.
Several pathogenic theories have been suggested for extrarenal WT (3). Some authors believe that extrarenal WT originates from Teratomas. Although in new classifications teratoid WT is distinguished from pure extrarenal WT. True primary extrarenal WT should lack teratoid elements. The most wildly accepted pathogenic hypothesis is the oncogenic transformation of ectopic nephrogenic rests to develop WT (3).
The diagnosis is based on pathologic features (4). Classic WT is composed of three blastemal, mesenchymal, and epithelial components. Although monophasic or biphasic tumors are not infrequent. The tumor cell nuclei are positive for WT1, in more than 90% and Glypican-3 is positive in the majority of the cases. CK, EMA, CD56 and CD57 are variably positive in epithelial component (5).
Carcinosarcoma is the most important differential diagnosis in uterus WT, especially among old patients. Lack of high nuclear features in both epithelial and stromal components, presence of primitive tubular and glomeruloid structures in epithelial elements, diffuse strong staining for WT1 and Glypican-3 and negative staining for ER/PR, as well as normal expression of p53 on IHC study, are all supportive of the WT diagnosis.
Mesonephric/ Mesonephric-like carcinoma is another differential diagnosis for uterus WT. Mesonephric/ Mesonephric-like carcinoma may show different morphologic patterns including papillary, ductal, solid, and spindle cell structures with mild to moderate nuclear atypia (22). Small tubular structures containing eosinophilic material and characteristic nuclear features resembling papillary thyroid carcinoma, were not identified in our case. GATA3, and TTF1, were also negative in the IHC study. Presence of leaf-like structures, peri-glandular cuffing, and minimal atypia in epithelial and spindle cell component, mimic Mullerian adenosarcoma (Figure 1A). Lack of immune reaction with ER and PR in epithelial cells and the presence of primitive tubular and glomeruloid structures are helpful in excluding this differential diagnosis.
There is no specific serum marker for the diagnosis of WT. Increased serum erythropoietin, Neuron-specific enolase, Carcino-eberyonic antigen, renin, and hormone tumor markers have been reported in WT. Elevated serum AFP is an unusual finding, which has been reported in a few cases of pediatric renal WT (7-10). Teratoid WT is expected to show increased AFP due to the presence of heterologous elements. However, as discussed earlier, Teratoid WT is not classified as primary WT (3, 23). In addition to the yolk sac and hepatic tumors, some other germ cell tumors including teratoma and somatic malignancies of gastrointestinal, lung, and breast could be associated with AFP secretion (24). In epithelial tumors of the female genital tract, increased AFP has been reported in different histologic subtypes with yolk sac or hepatoid differentiation and small cell neuroendocrine carcinoma (25-27). Our case was rigorously investigated with imaging studies and GI consultation to exclude the possibility of hepatic tumors. This is the first case report of extrarenal WT of the uterus with elevated AFP.
There is no consensus about treatment, follow-up, and prognosis of adult and especially uterine WT (28). Most of the previously reported cases and our case underwent hysterectomy. Only one case underwent chemotherapy as the initial treatment, which could not save the patient.
5.1. Conclusions
Uterine Wilm’s tumor is an extremely rare malignancy, which could be associated with elevated serum AFP. Diagnosis and treatment of this rare tumor is challenging for both pathologists and clinicians. Therefore, uterine WT should be considered as a differential diagnosis in malignant uterine tumors to help accurate diagnosis and future treatment planning.