1. Background
Endometrial cancer (EC) is the most common gynecologic malignancy in developed countries (1). Additionally, in 2018, EC was the second most common and fourth leading cause of gynecological cancer-related deaths globally (2). Recent data from the International Agency for Research on Cancer indicates a significant rise in EC incidence rates, with a projected global increase exceeding 50% by 2040 (1). Disparities in EC incidence and mortality are observed globally, with higher rates in high-income countries. The prevalence of modifiable risk factors, including tobacco use, unhealthy diets, and physical inactivity, is significantly higher in high-income countries, driving up EC incidence. Moreover, advanced healthcare systems and screening programs in these countries facilitate earlier detection and diagnosis, contributing to higher incidence rates. Conversely, lower access to healthcare and treatment options in low-income countries leads to higher mortality rates. Socioeconomic factors such as education and income levels, also play a crucial role in shaping EC incidence and mortality, particularly in low-income countries where limited access to healthcare and healthy lifestyle options exacerbates the issue (3, 4).
Risk factors of EC are obesity, radiation exposure, early menarche, late menopause, infertility, hormonal therapy use, older age, family history, and smoking (5-10). Results of an umbrella review showed that BMI and waist-to-hip ratio were strongly associated with an elevated risk of cancer and parity was associated with a strong reduction in the risk of the disease (11).
Currently, there is no specialized test available for evaluating EC. Most guidelines recommend transvaginal ultrasound or endometrial biopsy as the initial steps for evaluating EC (12, 13). Histological tissue changes in examining and staging these types of cancers can be informative (13). Metastasis plays a crucial role in the progression of cancers leading to mortality. During the metastatic process, cancer cells migrate through blood vessels and infiltrate other tissues, ultimately affecting healthy tissues. One intervening phenomenon in this process is angiogenesis. Angiogenesis, the formation of new blood vessels from existing ones, is essential in various pathological conditions such as tumor growth and metastasis, rheumatoid arthritis, as well as in physiological processes like organ growth, wound healing, and reproduction. In adults, subtle changes occur in endothelial cells, meaning these cells are dormant in maturity but have the capability to activate in response to appropriate factors. In other words, angiogenesis can be considered a necessary physiological process in the body that is regulated by a balance between inducers and inhibitors of angiogenesis. If this balance is disrupted, it provides a groundwork for the occurrence of certain diseases, including tumor growth and metastasis. Hence, understanding the factors involved in normal and abnormal angiogenesis is crucial and vital (14-16).
The extent of tumor angiogenesis, typically assessed through microvessel density (MVD), is commonly evaluated using antibodies targeting endothelial cells of blood microvessels, including factors VIII, CD31, CD34, and CD105 (17). Investigating MVD as an indicator of angiogenesis status can contribute to a better understanding of the diverse nature of these tumors and predict the occurrence of invasive or metastatic behavior in tumors. It can also aid in selecting treatment methods based on the differential expression of angiogenesis in various types of tumors.
2. Objectives
Hence, the present study was conducted with the aim of determining MVD using the CD31 marker and its associated factors in individuals with endometrial malignancies.
3. Methods
This cross-sectional study was conducted on 118 patients with EC who were referred to the pathology blocks of Imam Hussein Educational and Medical Center affiliated with Shahid Beheshti University of Medical Sciences, Tehran, Iran over a five-year period from 2018 to 2023. The study protocol received ethical approval from the Ethics Committee of Shahid Beheshti University of Medical Sciences (ethic code: IR.SBMU.RETECH.REC.; approval date: May 2023).
The inclusion criteria for participation in the study were having a complete medical and treatment history along with a definitive diagnosis of EC based on pathological reports. We employed a researcher-made checklist to gather data from the medical files of the patients. Data collected included patients age, BMI, gravidity, parity, history of abortion or still birth, delivery type, history of chronic disease, type of chronic disease, and factors related to tumor including invasion depth (no, less than 50%, more than 50%), lymph node involvement, grade of tumor, tumor type, metastasis, cancer stage, and patient’s outcome status (alive/ death). In this study, for the quantitative evaluation of the angiogenesis process, the endothelial marker CD31 was utilized as an immunohistochemistry marker to assess MVD. The advantages of using CD31 as a marker for MVD are: CD31 is a member of the immunoglobulin superfamily and is expressed on the surface of endothelial cells, making it a reliable marker for identifying blood vessels. In addition, CD31 is a sensitive marker, and CD31 expression can be easily detected using immunohistochemistry (IHC) techniques on formalin-fixed, paraffin-embedded tissue samples. This makes it a practical and accessible method for MVD assessment.
The appropriate sections for immunohistochemistry staining of the CD31 marker were determined by examining Hematoxylin and Eosin (H&E) stained slides as follows: Thin sections with a thickness of 3 microns were prepared initially. Immunohistochemistry staining for CD31 was then performed using Monoclonal mouse anti-human CD31 antibody (clone JC70A) from Dako, Denmark, at a dilution of 1:20 to 1:40 with Tris buffer at pH = 9. Quality control, including negative and positive controls, was conducted for each work series. Subsequently, the respective slides were initially scanned at low magnification (100x) to evaluate the areas with the maximum color receptivity. Then, at high magnification (400x), three areas with the maximum reactivity were examined, and the average count of small vessels (MVD) was estimated for each field.
3.1. Statistical Analysis
The data was analyzed using Stata software version 17. Quantitative baseline variables for patients were expressed as mean ± SD, while qualitative variables were presented using frequency and percentage. The comparison of MVD-CD 31 among tumor characteristics was performed using the student t-test and ANOVA. Crude and adjusted linear regression models were employed to identify predictors of MVD-CD 31 in patients with EC. A P-value less than 0.05 was considered as the significance level.
4. Results
In the current study, a total of 118 patients diagnosed with EC were studied. The mean age of the patients was 57.35 ± 11.16 years, with a range of 29 to 87 years. The BMI among the patients was 32.27 ± 5.17 kg/m2. Among the participants, 93 had a history of gravidity, and the average gravidity rate was 3.65 ± 2.41 (ranging from 1 to 13), as indicated in Table 1. Additionally, 15 cases reported a history of abortion, and stillbirth occurred in 12 patients. Out of the total, 58(49.15%) patients had hypertension, while 35 (29.66%) individuals had diabetes.
| Variable | Number | Mean ± SD | Range |
|---|---|---|---|
| Age (y) | 118 | 57.35 ± 11.16 | 29 - 87 |
| BMI (kg/m2) | 118 | 32.27 ± 5.17 | 22.7 - 53.6 |
| Gravidity | 93 | 3.65 ± 2.41 | 1 - 13 |
| Parity | 91 | 3.35 ± 2.1 | 1 - 10 |
| Live birth | 90 | 3.19 ± 2.02 | 1 - 10 |
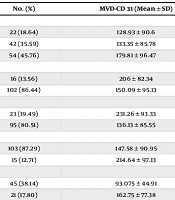
A total of 54 (45.76%) patients had an invasion depth of more than 50%. Lymph node engagement was observed in 16 (13.56%) patients, while metastasis to other organs occurred in 23 (19.49%) cases. Tumor grades 1, 2, and 3 were present in 38.14%, 17.8%, and 44.07% of patients, respectively. Regarding the cancer stage, 49.15% were classified as IA, with only 3 cases reaching stage IV. More than half of the cases featured low-grade endometrioid carcinoma, while 31.62% presented high-grade endometrioid carcinoma. Table 2 shows the mean values of MVD-CD31 of the patients according to tumor characteristics in endometrial lesions. Overall, the mean MVD-CD31 in the studied patients was 157.06 ± 94.31 (range, 32 385). Statistically significant differences were noted in the mean MVD-CD31 values based on invasion depth, ranging from 128.93 ± 90.6 in patients with no invasion depth to 179.81 ± 96.47 in those with over 50% invasion depth (P=0.04). Patients with metastasis exhibited a significantly higher mean MVD-CD31 (231.26 ± 93.33 vs. 136.13 ± 85.55, P < 0.001). Tumor grade increase correlated with a significant rise in the mean MVD-CD31 (P < 0.001). Concerning the cancer stage, stages IV and III showed significantly higher mean MVD-CD31 values of 261.67 ± 107.28 and 211.06 ± 94.05, respectively (P=0.007). Clear cell carcinoma and serous carcinoma types demonstrated significantly higher mean MVD-CD31 values (P < 0.001).
| Tumor Characteristics | No. (%) | MVD-CD 31 (Mean ± SD) | P-Value |
|---|---|---|---|
| Invasion depth | 0.04 | ||
| No | 22 (18.64) | 128.93 ± 90.6 | |
| Less than 50% | 42 (35.59) | 133.35 ± 85.78 | |
| More than 50% | 54 (45.76) | 179.81 ± 96.47 | |
| Lymph node engagement | 0.06 | ||
| Yes | 16 (13.56) | 206 ± 82.34 | |
| No | 102 (86.44) | 150.09 ± 95.13 | |
| Metastasis | < 0.001 | ||
| Yes | 23 (19.49) | 231.26 ± 93.33 | |
| No | 95 (80.51) | 136.13 ± 85.55 | |
| Outcome status | 0.013 | ||
| Alive | 103 (87.29) | 147.58 ± 90.95 | |
| Death | 15 (12.71) | 214.64 ± 97.13 | |
| Tumor grade | < 0.001 | ||
| I | 45 (38.14) | 93.075 ± 44.91 | |
| II | 21 (17.80) | 162.75 ± 77.38 | |
| III | 52 (44.07) | 214.46 ± 97.24 | |
| Cancer stage | 0.007 | ||
| IA | 58 (49.15) | 131.04 ± 85.82 | |
| IB | 23 (19.48) | 148.61 ± 86.42 | |
| II | 8 (6.78) | 173.5 ± 102.92 | |
| III | 26 (22.03) | 211.06 ± 94.05 | |
| IV | 3 (2.54) | 261.67 ± 107.28 | |
| Tumor type | < 0.001 | ||
| Low-grade endometrioid carcinoma | 62 (52.99) | 113.58 ± 64.24 | |
| High-grade endometrioid carcinoma | 37 (31.62) | 202.69 ± 102.7 | |
| Clear cell carcinoma | 5 (4.27) | 348 ± 32.53 | |
| Serous carcinoma | 10 (8.55) | 219.38 ± 79.49 | |
| Un-differentiated carcinoma | 3 (2.56) | 182.5 ± 24.75 |
The crude and adjusted models for predictors of MVD-CD31 in EC patients are presented in Table 3. Results of the multivariable linear regression model showed that after adjusting for other variables in the model, the mean value of MVD-CD31 in patients with more than 50% invasion depth, 79.59 is higher than in patients with no invasion depth (P = 0.003). Moreover, lymph node engagement and metastasis to other organs were significantly associated with higher MVD-CD 31 amount (P < 0.001). Compared tumor grade 1, patients with grade 2 tumor had higher amount of MVD-CD 31 (Mean difference: 64.85, P = 0.007). Finally, patients with clear cell carcinoma tumor type had significantly higher amounts of MVD-CD 31 compared to patients with low-grade endometrioid carcinoma tumor type (Mean difference: 225.84, P = 0.005).
| Variables | Crude Model | Adjusted Model | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | P-Value | β | 95% CI | P-Value | |
| Age (y) | 3.33 | 1.66, 5.01 | < 0.001 | |||
| BMI (kg/m2) | 0.84 | -3.17, 4.84 | 0.68 | |||
| Gravidity (number) | 6.78 | -1.8, 15.36 | 0.12 | -6.84 | -15.66, 1.99 | 0.13 |
| Follow-up time (per six month) | 15.43 | 1.44, 29.43 | 0.03 | 10.54 | -4.55, 25.63 | 0.17 |
| Hypertension | ||||||
| No | Reference | |||||
| Yes | 12.16 | -25.57, 49.9 | 0.52 | |||
| Diabetes | ||||||
| No | Reference | |||||
| Yes | -7.42 | -49.85, 35.01 | 0.73 | |||
| Invasion depth | ||||||
| No | Reference | Reference | ||||
| Less than 50% | 4.42 | -53.6, 62.43 | 0.88 | 42.65 | -9.04, 94.33 | 0.1 |
| More than 50% | 50.88 | -4.6, 106.37 | 0.07 | 79.59 | 28.23, 130.95 | 0.003 |
| Lymph node engagement | ||||||
| No | Reference | 0.059 | Reference | < 0.001 | ||
| Yes | 55.91 | -1.94, 113.75 | 90.57 | 27.09, 154.06 | ||
| Metastasis | ||||||
| No | Reference | < 0.001 | Reference | < 0.001 | ||
| Yes | 95.13 | 50.02, 140.24 | 103.28 | 43.37, 163.18 | ||
| Survival status | ||||||
| Alive | Reference | 0.013 | ||||
| Death | 67.07 | 14.51, 119.62 | ||||
| Tumor grade | ||||||
| I | Reference | Reference | ||||
| II | 69.98 | 24.61, 114.74 | 0.003 | 64.85 | 18.4, 111.3 | 0.007 |
| III | 121.39 | 87.92, 154.86 | < 0.001 | 57.006 | -49.15, 163.16 | 0.29 |
| IA | Reference | |||||
| Cancer stage | ||||||
| IB | 17.57 | -27.6, 62.72 | 0.44 | |||
| II | 42.45 | -25.4, 110.32 | 0.22 | |||
| III | 80.02 | 30.83, 129.19 | 0.002 | |||
| IV | 130.62 | 24.96, 236.28 | 0.016 | |||
| Tumor type | ||||||
| LGEC | Reference | Reference | ||||
| HGEC | 89.11 | 53.96, 124.25 | < 0.001 | 17.6 | -85.21, 120.71 | 0.73 |
| CCC | 234.42 | 120.64, 348.2 | < 0.001 | 225.84 | 71.09, 380.58 | 0.005 |
| SC | 102.79 | 45.98, 165.60 | 0.001 | 129.97 | -20.24, 280.18 | 0.088 |
Abbreviations: LGEC, low grade endometrioid carcinoma; HGEC, high grade endometrioid carcinoma; CCC, clear cell carcinoma; SC, serous carcinoma.
5. Discussion
The current study was conducted to investigate MVD utilizing the CD31 marker and its associated factors in patients diagnosed with endometrial malignancies. Our findings indicate that some tumor characteristics such as invasion depth, lymph node involvement, tumor grade, and tumor type are associated with an increase in MVD-CD31 levels among EC patients.
In line with our results, Kilinc and Bahar's study demonstrated significant associations between intratumoral and extratumoral MVD and deep myometrial invasion, high grade, non endometrioid tumor type, cervix invasion, lymph node metastasis, advanced stage (III to IV), substantial lymphovascular invasion, and overall survival (18). Tumor growth and metastasis hinge on the crucial process of angiogenesis. Research have indicated that tumors with larger diameters display elevated densities of microvessels, promoting enhanced blood flow perfusion. This phenomenon sustains invasive growth and disrupts surrounding tissues (18). Additionally, in the study conducted by Landt et al. a distinct association was observed between the concentrations of angiogenic factors and the stage of disease, and the invasive stages of EC (19). Tumors require a blood supply to support their growth and provide essential nutrients. When cancer cells invade surrounding tissues extensively (invasion depth exceeding 50%), the body's response may include an increased formation of new blood vessels (angiogenesis) to meet the growing demands of the tumor (20). The majority of EC cases are typically detected in their early stages. However, approximately 15 - 20% of women diagnosed with aggressive cancer types face an elevated risk of hidden malignant spread and tumor recurrence, even after undergoing chemotherapy and radiotherapy (21). The primary approach for categorizing EC cases into prognostic groups, guiding the selection of various surgical and chemo- or radiotherapeutic interventions, is the utilization of tumor staging based on the FIGO criteria. There has been a growing focus on understanding the factors that associated with the growth of EC and its interactions within the adjacent uterine stromal microenvironment. Currently, our knowledge regarding the regulation of tumor budding (TB) and MVD in EC remains limited. Moreover, in various examined cancer types, including lung, breast, colorectal, and endometrial endometrioid cancers, the presence of the TB phenomenon has consistently been linked to poor survival rates (22, 23).
In regards of tumor type, in our study patients with clear cell carcinoma tumor type had significantly higher amount of MVD-CD 31. In fact, the highest MVD values are identified at the invading tumor edge, exhibiting a density that can be 4 - 10 times higher than within the tumor interior. Furthermore, the organization of vessels in the central region of the tumor is notably more disordered compared to the more structured patterns observed at its periphery (24).
Our findings have important implications for the clinical management of EC. The identified associations between tumor characteristics (invasion depth, lymph node involvement, tumor grade, and type) and MVD highlight the potential prognostic significance of angiogenesis levels. Clinicians may consider integrating assessments of these characteristics into diagnostic and treatment decision-making processes. While anti-angiogenic agents, as well as PI3kinase/mTOR and MEK inhibitors, have shown activity, the conclusive evidence of their benefits remains inconclusive. This uncertainty stems from the restricted sample size of trials, inconsistencies in results, and the drugs' low therapeutic index. Consequently, there is a need for further investigations through well-designed and adequately powered molecularly driven randomized trials to establish a more comprehensive understanding of their efficacy (25).
Nevertheless, the present study is subject to several limitations. First, the study was conducted at a single center, limiting the generalizability of the findings to a broader population and results may not be representative of variations in EC characteristics across different geographical or healthcare settings. Secondly, due to retrospective data collection from medical records, the accuracy of results is dependent on the quality and completeness of historical patient records. Thirdly, the small sample size of the study, potentially limiting the statistical power of the analyses. Additionally, the use of CD31 as the sole marker for evaluating MVD might overlook other relevant markers. The complexity of angiogenesis may require a more comprehensive panel of markers for a thorough assessment. Finally, despite adjusting for various factors in the multivariable regression model, there may be unmeasured confounding variables such as treatment modality may influence the observed associations.
5.1. Conclusions
Our results suggested that some tumor characteristics such as invasion depth, lymph node involvement, tumor grade, and tumor type may play a role in angiogenesis in patients with EC. These findings suggest that tumor features play a crucial role in modulating angiogenesis in EC. Understanding these relationships may contribute to the development of targeted therapeutic approaches and prognostic assessments for patients with EC. Further research and validation studies are warranted to deepen our understanding of these associations and their implications for clinical management.