1. Introduction
Endometriosis, a condition characterized by endometrial glands and stroma outside the uterus, was initially described by Rokitansky in 1860 (1). It affects approximately 6% to 10% of women of reproductive age, which translates to around 190 million women worldwide, and is associated with significant healthcare costs (2, 3). While endometriosis primarily affects pelvic organs, it can also be found in extra pelvic organs and tissues (4). However, extra-pelvic endometriosis is relatively rare, accounting for less than 12% of reported cases (5). Although most cases of endometriosis are benign, a small percentage (0.5% to 1%) can develop into malignancy (6). Dr. Sampson first described the transformation of endometriosis into malignancy in 1925. Malignant tumors originating from endometriosis are typically endometrioid adenocarcinoma and clear cell types, with extremely rare cases of endometrial stromal sarcoma (ESS) (7, 8). Endometrial stromal sarcoma is a rare form of uterine sarcoma, comprising approximately 0.2% of all uterine malignancies and 10% to 15% of all uterine sarcomas (9). It arises from the invasive proliferation of cells resembling stromal cells of the normal proliferative endometrium, primarily in the uterine corpus but can also originate from extrauterine sites (9). The majority of extrauterine ESS cases result from the malignant transformation of endometriosis, which occurs in only 0.1% to 0.7% of cases (10). Endometrial stromal sarcoma is often misdiagnosed as benign conditions, such as leiomyoma, and is typically confirmed through post-operative histopathological evaluation (11). The exact pathogenesis of ESS is still not fully understood but identified risk factors include past exposure to pelvic radiation therapy, long-term tamoxifen use, and unopposed estrogen use (12). The World Health Organization (WHO) categorizes ESS into low- and high-grade forms, as well as nodular and undifferentiated variants, providing a comprehensive classification system for this neoplasm (13). Different subtypes of endometrial sarcoma have distinct therapeutic and prognostic characteristics. High-grade and undifferentiated endometrial sarcoma, referred to as high-grade ESS, is known for its aggressive behavior and has a 5-year survival rate of only 33%. On the other hand, low-grade endometrial stromal sarcoma (LG-ESS) behaves more indolently and is associated with a 5-year survival rate of 91% (14, 15). Low-grade endometrial stromal sarcoma is a rare form of cancer that originates from the mesenchymal cells in the endometrium. It accounts for only 0.2% of all gynecological malignancies and has an annual occurrence of 2 cases per million women worldwide. Several risk factors have been associated with LG-ESS, including obesity, diabetes mellitus, and prolonged exposure to hormones such as early onset of menstruation, late menopause, and hormonal treatments. Among these risk factors, it has been found that prolonged use of tamoxifen or pelvic radiation therapy are the most significant contributors to the development of LG-ESS (16, 17). Typically, the clinical manifestation of this condition involves the presence of non-specific symptoms, including chronic vaginal bleeding, pelvic pain, and dysmenorrhea (18). Low-grade endometrial stromal sarcoma is typically found in the uterus but can also occur in extrauterine sites, known as LG-EESS. The incidence of LG-EESS is even rarer and not well-documented. LG-EESS is believed to stem from ectopic endometrial stroma, which may account for its close association with endometriosis. A review of literature published between 1970 and 2016 on PubMed identified 76 cases of extrauterine endometrial stromal sarcoma (EESS) originating from endometriosis. The most commonly involved sites were the ovary (44.3%), pelvic organs outside the uterus and ovaries (15.2%), sigmoid rectum (7.6%), and small intestine (7.6%) (19). Extrauterine endometrial stromal sarcoma arising in the peritoneum is a rare occurrence, and as a result, only a few studies have reported such cases (20, 21). In previous reports, the age of the patients was over 42 years old and usually in menopause. Furthermore, previous reports have not described the radiological features of the disease in a precise manner. This case report presents a 35-year-old female who initially presented with pelvic pain and was diagnosed with disseminated peritoneal endometriosis on multiple occasions. Five years after the initial diagnosis, the patient developed LG-EESS. This case is noteworthy due to the patient's young age at diagnosis and the rarity of the disease. To the best of our knowledge, there are only a few reports that describe the radiological features of an LG-EESS originating from the peritoneum, particularly in the context of deep infiltrating endometriosis without involvement of the endometrial cavity.
2. Case Presentation
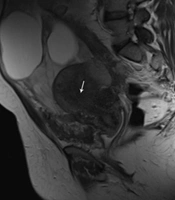
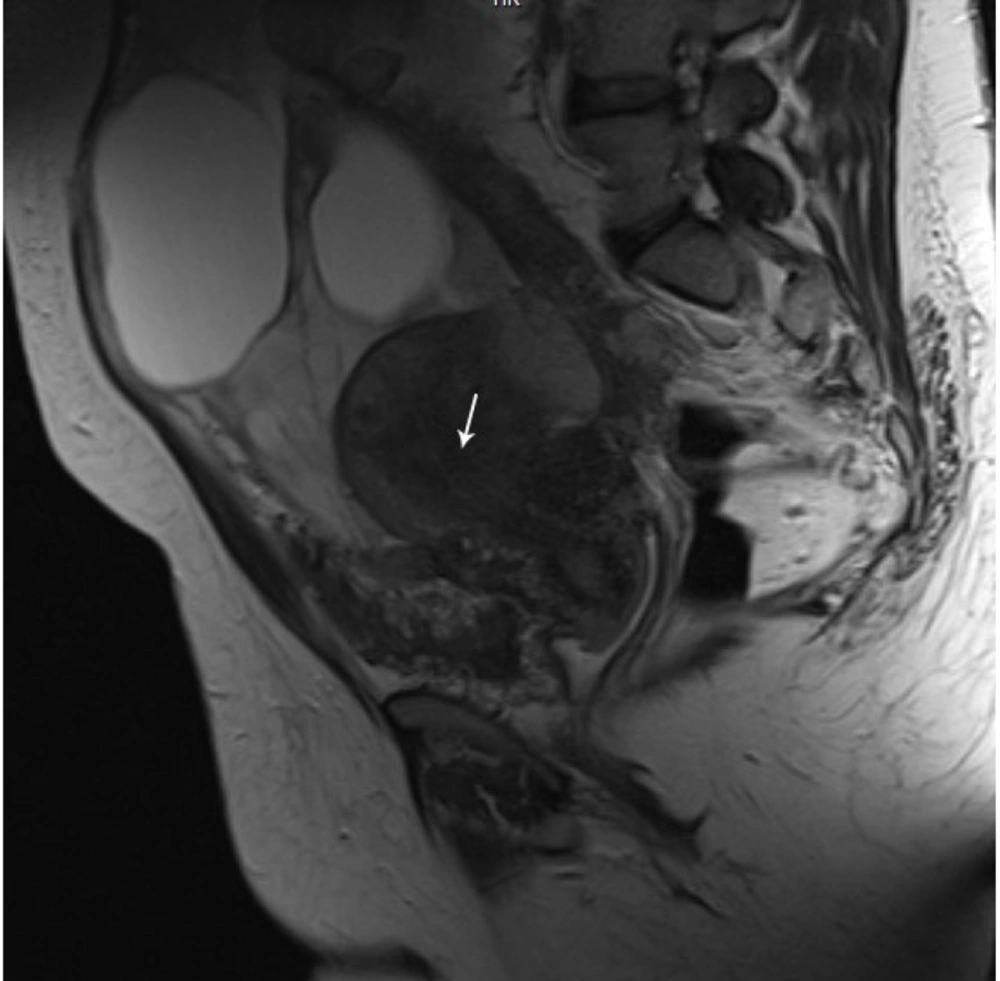
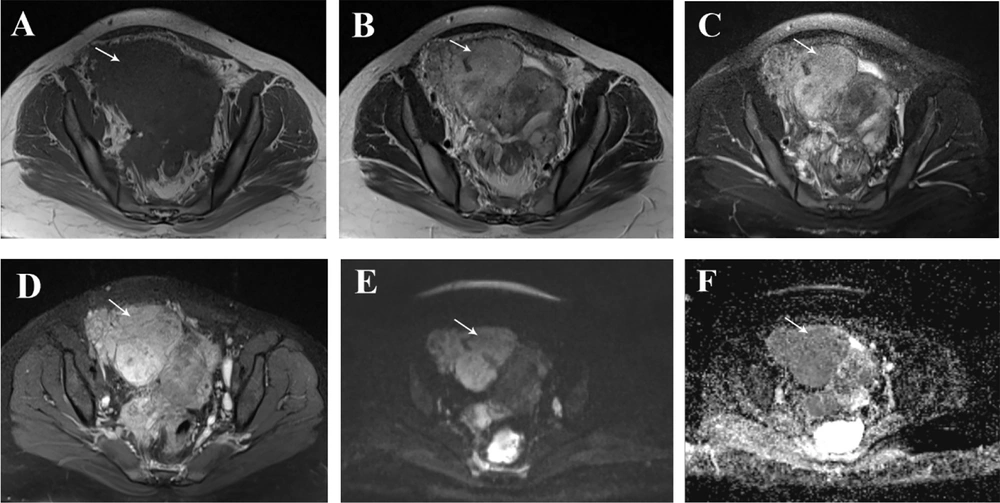
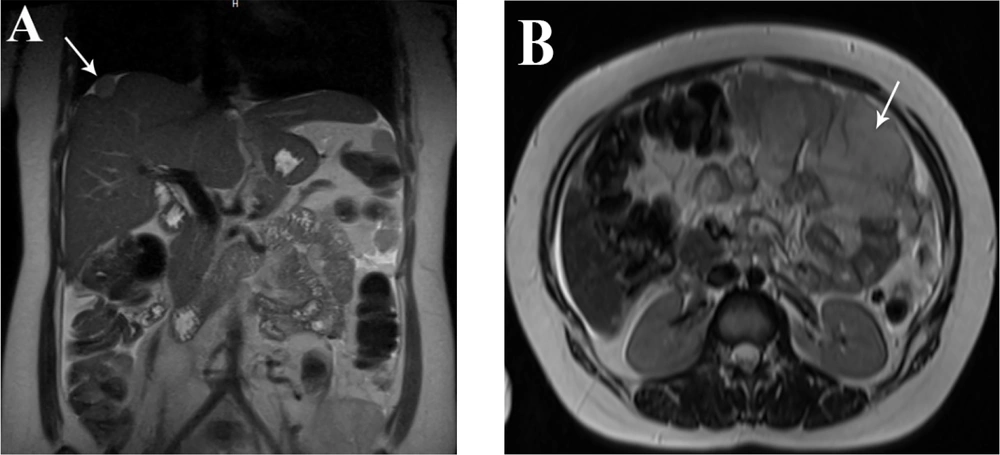
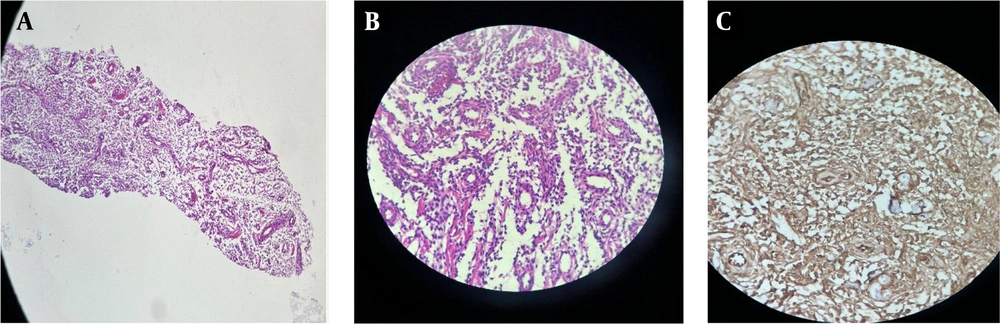
A 35-year-old woman who had previously been diagnosed with stage IV endometriosis five years ago visited her gynecologist with complaints of chronic pain in the abdomen and pelvis, as well as dysmenorrhea. The gynecologist, upon receiving the referral, ordered a transvaginal ultrasound (US) and a magnetic resonance imaging (MRI) for further evaluation. The ultrasound revealed the presence of multicystic structures with mural nodules in both tubo-ovarian complexes. Subsequently, the abdominopelvic MRI, with and without intravenous gadolinium, showed severe adhesions surrounding the uterus in both tubo-ovarian complexes. The ovaries appeared to be absent, while the uterus was observed in its normal size and anterior position. Additionally, the MRI indicated evidence of adenomyosis, characterized by irregular thickening of the junctional zone in the uterus. The endometrial-myometrial junction appeared ill-defined and unsharp (Figure 1). Furthermore, there was thickening of the uterosacral ligament in the posterior aspect of the uterus, along with the presence of endometriotic nodules in the pouch of Douglas and torus uterinus, as well as the attachment of the uterosacral ligament. Cystic lesions displaying hypersignal without internal enhancement, indicating the possibility of endometrioid cyst, were observed in both tubo-ovarian complexes. The measurements revealed a size of 125 × 66 mm on the right side and 104 × 67 mm on the left side. Additionally, solid lesions with T1-Weighted iso signal and T2-Weighted hypersignal, along with pathologic enhancement and diffusion restriction, were detected surrounding the tubo-ovarian complexes, uterus, right para rectal area, rectosigmoid junction, right pelvic side, extending into the abdominal cavity, and involving the peritoneum up to the sub hepatic level. These findings suggest the presence of metastatic implantation (Figures 2 and 3). Following a multidisciplinary meeting, the gynecologist opted to perform a core needle biopsy on the largest lesion of the peritoneum, measuring 118 × 72 mm. Upon histopathological examination, the sections revealed fibroadipose tissue infiltrated by neoplastic proliferation of spindle to oval-shaped cells with a bland appearance, arranged concentrically around small vessels. These cells exhibited minimal cytoplasm and mild nuclear atypia, with no observed mitotic figures. The specimen did not contain endometrial glands or hemosiderin-laden macrophages, and there was an absence of tumor necrosis or vascular invasion. Based on the morphological characteristics observed, the diagnosis was interpreted as LG-EESS (Figure 4A, B). Therefore, immunohistochemistry stains (IHC) were recommended to confirm the diagnosis. Immunohistochemistry stains showed strong positivity for CD10 (Figure 4C). This panel supported the diagnosis of LG-EESS, and the patient was referred for oncology consultation. Written informed consent was obtained from the patient before proceeding.
Pelvic magnetic resonance imaging (MRI), axial sequences: T1- Weighted iso signal (A), T2- Weighted hypersignal (B) and T2 fat saturated hypersignal (C) solid lesions with pathologic enhancement in T1 fat saturated post-contrast (gadolinium) sequence (D), diffusion restriction (presence of high signal intensity on diffusion-weighted imaging (DWI) sequence (E) and low signal intensity on afferent diffusion coefficient map (ADC) (F) is seen at the right pelvic side with extension to abdominal cavity suggestive for metastatic implantation.
Abdominal magnetic resonance imaging (MRI) scan (T2- weighted image): In the coronal plane (A) a solid lesion with an intermediate to high signal in the T2 sequence and a brief retraction in the liver capsule (liver dome) is seen, which demonstrates capsular implantation and in the axial plane (B) solid lesion with t2 high signal intensity that involving the peritoneum and suggesting metastatic lesions.
Histopathology of the peritoneal lesion core needle biopsy: A, shows LG-ESS with small vessel proliferation (H&E 10 ×); B, monotonous oval-shaped cells are concentrically arranged around small vessels (H&E 40 ×); C, immunohistochemical features of the tumor show membranous positivity for CD 10 (40 ×).
3. Discussion
The symptoms of ESS and endometriosis are similar and depend on the site of involvement. LG-EESS arising from endometriosis is an extremely rare condition, reported only in a few case reports (19, 22). The majority of literature sources primarily have focused on the clinical and pathological aspects of LG-EESS, with little attention given to the radiological findings associated with this condition (14, 23). Diagnosing EESS preoperatively can be difficult, particularly when uterine ESS is not present, as in this particular case. This challenge arises due to the rarity of the condition and the radiological resemblance it shares with other tumors like leiomyoma, leiomyosarcoma, and gastrointestinal stromal tumors.
3.1. MRI Imaging Characteristics of Low-grade Endometrial Stromal Sarcoma and Low-grade Extrauterine Endometrial Stromal Sarcoma
From a radiological perspective, uterine LG-ESS typically manifests as a polypoidal endometrial mass with either a well-defined or infiltrative border (15). magnetic resonance imaging commonly demonstrates mixed signal intensity on T1-weighted images and mixed high signal intensity on T2-weighted images, with potential variations in T2 signal intensity based on degenerative alterations. Distinctive MRI characteristics of LG-ESS include a worm-like nodular projection suggesting infiltration of lymphatic and vascular structures into the myometrium, as well as hypointense bands on T2-weighted images representing intact normal myometrium encircled by the tumor, along with extension along ligaments; LG-ESS can be identified through specific MRI findings such as nodular projections resembling worms, hypointense bands on T2-weighted images indicating normal myometrium preservation within the tumor, and extension along ligaments (24-26). Following contrast enhancement, LG-ESS typically exhibits a moderate level of enhancement, while concurrently displaying restricted diffusion characterized by a mean ADC value of 1.09 (27). The reported SUVmax of LG-ESS was 5.41 and 13.28 (28, 29). There is a scarcity of reports regarding the imaging characteristics of LG-EESS due to the infrequency of these tumors. In current case report, the presence of extensive solid lesions in the pelvis and peritoneum, measuring over 10 cm in the peritoneum, along with alterations in signal intensity on T2 high images, diffusion restriction, and enhancement in post-contrast images, raised concerns about the potential malignancy of these lesions. Consequently, the patient was deemed suitable for a biopsy to confirm the definitive diagnosis, as imaging techniques alone do not offer distinct indicators for ESS (30).
3.2. Pathological Findings of Low-grade Extrauterine Endometrial Stromal Sarcoma
Pathological findings of LG-EESS are similar to those of uterine LG-ESS (31). Endometrial stromal sarcoma typically presents as a neoplasm that bears resemblance to the proliferative phase endometrial stroma. The tumor exhibits a diffuse architecture and consists of monomorphic cells with oval to spindle-shaped nuclei. The mitotic count varies, and this criterion is no longer considered in the current classification of tumors of female reproductive organs by the WHO. The tumor stroma is characterized by a well-developed vascular network composed of small vessels, some of which have hyalinized walls resembling endometrial spiral arterioles. The tumor cells often form a whorling pattern around these vessels. In some cases, ESS may exhibit unusual features such as smooth muscle differentiation, a myxoid background, a fibroblastic appearance, calcifications, epithelioid differentiation, sex cord-like differentiation, and clear cell differentiation. The tumor borders are typically irregular and may show vascular invasion as well as tongue-like projections into the vessel walls (32, 33). In line with prior research, the histopathological examination of the peritoneal lesion core needle biopsy in our case revealed a consistent pattern of monotonous oval-shaped cells that were arranged in a concentric manner around small blood vessels, which is indicative of LG-EESS. On a macroscopic level, LG-ESS appears as a well-defined, solid tumor with multiple nodules. The tumor is characterized by the presence of monotonous cells that exhibit limited cytoplasm and minimal abnormality (14).
3.3. Immunohistochemistry Markers of Low-grade Extrauterine Endometrial Stromal Sarcoma
CD10, Smooth Muscle Actin (SMA), Desmin, H-Caldesmon, Cytokeratin, and hormone receptors (estrogen and progesterone) are the most commonly used immunohistochemistry markers to differentiate ESS from other tumors. Among these markers, CD10 plays a crucial role in distinguishing LG-ESS from its histological mimics, particularly cellular leiomyoma. A significant diagnostic indicator, diffuse and strong positivity for CD10, aids in identifying LG-ESS, as opposed to cellular leiomyoma where CD10 is predominantly negative. This distinction is valuable in accurately diagnosing ESS and avoiding misinterpretation (16). In our study, we validated the diagnosis of LG-ESS based on the significant CD10 positivity. Not using other immunohistochemistry markers was a limitation of our study.
3.4. Therapeutic Approach for Low-grade Extrauterine Endometrial Stromal Sarcoma
Low-grade extrauterine endometrial stromal sarcoma is classified as a slow-growing tumor with a tendency for late recurrences, even though many patients initially present with advanced stages of the disease. The optimal therapeutic approach for EESS remains uncertain due to its rarity, but surgical intervention is generally recommended. In cases of advanced tumor stages, adjuvant therapy such as hormonal therapy, chemotherapy, or radiation therapy should be taken into account (30).
3.5. Conclusions
The occurrence of malignant transformation in endometriotic lesions is relatively infrequent, yet it is crucial to promptly identify them. This particular case emphasizes the significance of closely monitoring patients with endometriosis to enable the timely detection of any potential malignant transformations.