1. Background
Unusual uterine bleeding is a leading reason for gynecological clinic visits, accounting for approximately 20% of all consultations (1-3). It is often an early symptom of various pathologies, including polyps, submucosal myomas, hyperplasias, and, most critically, endometrial cancer (EC) (4-6). Therefore, any woman over 35 experiencing anovulatory uterine bleeding should undergo an endometrial examination to rule out malignancy.
In recent years, the incidence of EC has steadily risen, posing a significant threat to women's health (7-9). Due to its subtle early-stage symptoms, EC is frequently diagnosed at an advanced stage, reducing treatment efficacy and survival rates. A 2008 study reported EC prevalence at 1.7% in Iran compared to 8.2% globally (10). The EC ranks as the sixth most common cancer among women worldwide, with 319,000 new cases diagnosed in 2012 - 75% of which occurred postmenopause. Early-onset endometrial cancer (EOEC), diagnosed before age 50, remains relatively rare, with an incidence rate of 6.9 per 100,000 women in 2018.
Despite its prevalence, EC is often considered a “curable cancer” since 75% of cases are diagnosed before the disease spreads beyond the uterus. According to the International Federation of Obstetricians and Gynecologists (FIGO), the overall five-year survival rate for EC is approximately 86%, increasing to 97% if the cancer remains confined to the uterus (11-13).
Human epididymal protein 4 (HE4), encoded by the whey-acidic-protein (WAP) four-disulfide core domain protein 2 (WFDC2) gene, has emerged as a key tumor marker for epithelial ovarian cancer (14-16). Further research into HE4’s role in EC diagnosis and prognosis could enhance early detection and improve patient outcomes.
2. Objectives
This study was conducted to determine the serum level of HE4 and compare its levels in patients with abnormal bleeding due to benign uterine lesions with the group with lesions due to EC. This research also investigates the relationship between the serum level of this tumor and the grade of various malignancies that seem essential. Considering the importance of EC, the burden caused by the disease in the female population, and the limitations mentioned in the existing diagnostic methods, this study aimed to investigate HE4 in women with abnormal uterine bleeding (AUB).
3. Methods
3.1. Study Population
In this retrospective cohort study, 38 women with AUB between 35 - 65 years old between March 15, 2022, and September 15, 2023 were included in the data registry. Then, they underwent a biopsy along with measuring the serum level of HE4. To determine the role of HE4 in differentiating malignant and benign pathologies, 38 patients were studied. Human epididymal protein 4 levels were measured two weeks before surgical intervention. The HE4 cutoff point was considered to be less than 140 pmol/L.
Survival analysis was done to determine if there was a significant difference in the starting point, including the time from AUB to EC diagnosis as the endpoint.
Patients were divided into two groups based on the pathology report. The first group consisted of women whose pathology reports showed EC, and the second group of women whose pathology reports showed benign causes of AUB.
A complete medical history was obtained from all patients enrolled in the study, and their demographic information was recorded in a patient data collection sheet. Patients were then assessed for metastasis based on their clinical history, physical examination, and relevant paraclinical tests, as guided by their symptoms and complaints.
Inclusion criteria included all patients with AUB aged between 35 and 65 years who underwent a biopsy and had measured HE4 serum levels, while patients with cervical lesions were excluded.
Cox proportional hazards and the Kaplan-Meier models were used for the survival analysis of EC. Initially, Cox proportional hazards (PH) regression was employed, but the Schoenfeld residuals test revealed violations of the proportionality assumption for two variables: Human epididymal protein 4 level and EC diagnosis. Consequently, alternative accelerated failure time (AFT) models were utilized, including exponential, Weibull, log-normal, log-logistic, and generalized gamma distributions. The Kaplan-Meier estimator was used to calculate overall survival probabilities. Model selection was based on the Akaike information criterion (AIC) and graphical assessment via Cox-Snell residuals. Univariate analysis identified significant variables (P < 0.2), which were then included in a multivariate model, ultimately selecting the AFT distribution with the lowest AIC for further analysis.
In addition, the agreement tables and the odds ratio coefficient were calculated for this purpose. Multiple logistic regression tests were also used for prediction. The data were analyzed using the software program STATA version 12 (STATA Corporation, College Station, TX).
This research was approved by ethic committee with ethical codes: IR.MEDILAM.REC.1398.194, IR.MEDILAM.REC.1399.103.
Written informed consent was obtained from patients who agreed to participate in the study.
4. Results
The ages of participants in the EC and non-EC groups averaged 49.89 ± 10.8 and 45.47 ± 6.6 years, respectively. The average ages of menopausal and non-menopausal women were 55.36 and 40 years, respectively. Most of the EC patients had undergraduate and postgraduate education (33.9%), the majority were married (63.6%), and mostly housewives (62.5%). None of the patients in the study reported a history of smoking, alcohol, or drug use. Other patients' descriptive statistics are presented in Table 1.
| Variables | EC | Non-EC | Cox Proportional Hazard Ratio (95% CI) | P-Value b |
|---|---|---|---|---|
| Age (y) | 49.48 ± 10.8 | 45.47 ± 6.6 | 1.05 (1.02 - 1.06) | < 0.001 |
| Body Mass Index (kg/m2) | 24.3 ± 0.3 | 25.7 ± 0.1 | 0.98 (0.96 - 1.01) | 0.06 |
| Menopause | ||||
| No | 9 (47.4) | 10 (52.6) | 1 | - |
| Yes | 10 (52.6) | 9 (47.4) | 2.26 (1.62 - 3.15) | < 0.001 |
| Myometrial involvement (%) | ||||
| No | 6 (54.5) | 13 (48.1) | 1 | - |
| Yes | 5 (45.5) | 14 (51.9) | 1.73 (0.86 - 3.89) | 0.04 |
| History of infertility (%) | ||||
| No | 8 (64.9) | 15 (80.1) | 1 | - |
| Yes | 11 (35.1) | 4 (19.9) | 1.73 (1.38 - 2.18) | < 0.001 |
| Hypertension (%) | ||||
| No | 6 (54.9) | 14 (71.0) | 1 | - |
| Yes | 13 (45.1) | 5 (29.0) | 1.57 (1.26 - 1.96) | < 0.001 |
| Diabetes (%) | ||||
| No | 9 (67.0) | 16 (78.5) | 1 | - |
| Yes | 10 (33.0) | 3 (21.5) | 1.67 (1.17 - 1.85) | 0.001 |
| Autoimmune disease (%) | ||||
| No | 11 (96.1) | 15 (97.6) | 1 | - |
| Yes | 8 (3.9) | 2 (2.4) | 1.75 (1.00 - 3.05) | 0.05 |
| Malignancy (%) | ||||
| No | 12 (91.3) | 17 (98.3) | 1 | - |
| Yes | 7 (8.7) | 2 (1.7) | 1.01 (0.95 - 1.11) | < 0.001 |
| Grad | ||||
| 1 | 7 (36.8) | 11 (57.9) | 1 | - |
| 2 | 6(31.6) | 4 (21.1) | 1.86 (1.46 - 2.37) | |
| 3 | 6 (31.6) | 4 (21.1) | 1.47 (0.84 - 2.56) | 0.03 |
| HE4 (gr/dL) | 115.03 ± 31.01 | 78.01 ± 32.29 | 1.89 (1.25 - 2.04) | < 0.001 |
Cox Proportional Hazard Models of Factors for Endometrial Cancer a
In this study, the mean serum level of HE4 in menopausal and non-menopausal patients of the EC group was significantly higher than that of the group without EC. Also, the serum level was significantly higher in menopausal patients of the EC group than that of non-menopausal women in this group.
The results showed that the serum levels of HE4 were higher in all patients, menopausal and non-menopausal patients with myometrial involvement than that of patients without myometrial involvement (Table 2).
| Characteristics | EC = 19 (%) | Non-EC = 19 (%) | P-Value |
|---|---|---|---|
| No menopause | 26.13 ± 95 | 11.4 ± 57.02 | - |
| Menopause | 23.54 ± 133.06 | 32.22 ± 101.33 | 0.02 |
| Myometrial involvement | |||
| No | 32.10 ± 109.74 | 31.89 ± 73.77 | - |
| Yes | 27.53 ± 126.50 | 33.91 ± 89.86 | 0.153 |
| Degree of differentiation (FIGO) | |||
| I | 80.25 ± 7.3 | 69.38 ± 18.44 | - |
| II | 130.59 ± 17.75 | 64.85 ± 25.55 | 0.65 |
| III | 140.05 ± 18.62 | 114.90 ± 46.40 | - |
The Mean and Standard Deviation of Human Epididymal Protein 4 Serum Levels in the Studied Groups
Univariate analysis using the logarithmic distribution confirmed a significant association between HE4 and EC (TR: 1.85; 95% CI: 1.29 - 2.14; P < 0.001), indicating an increased outcome with increasing EC (Table 3).
| Variables | Time Ratio (95% CI) | P-Value |
|---|---|---|
| Age (y) | 1.03 (0.99 - 1.06) | < 0.001 a |
| Menopause | 2.36 (1.52 - 3.25) | < 0.001 a |
| Diabetes | 1.60 (1.27 - 1.89) | 0.04 |
| History of infertility | 1.70 (1.10 - 2.35) | < 0.001 a |
| Myometrial involvement | 1.63 (0.96 - 3.79) | 0.001 a |
| HE4 | 1.85 (1.29 - 2.14) | 0.05 a |
| Grad | 1.40 (0.94 - 2.50) | 0.05 |
| Hypertension | 1.54 (1.25 - 2.78) | 0.001 a |
| Cardiovascular disease | 1.79 (1.39 - 2.28) | < 0.001 a |
Analysis of Univariate Accelerated Failure Time Regression Models for Endometrial Cancer
Adjusted for other variables of the multivariate model, the adjusted TR for HE4 was 1.89 (95% CI: 1.30 - 2.19; P < 0.001; Table 4).
Multivariate Analysis Using the Weibull Accelerated Failure Time Model for the Effect of Human Epididymal Protein 4 Levels and Other Factors on Endometrial Cancer
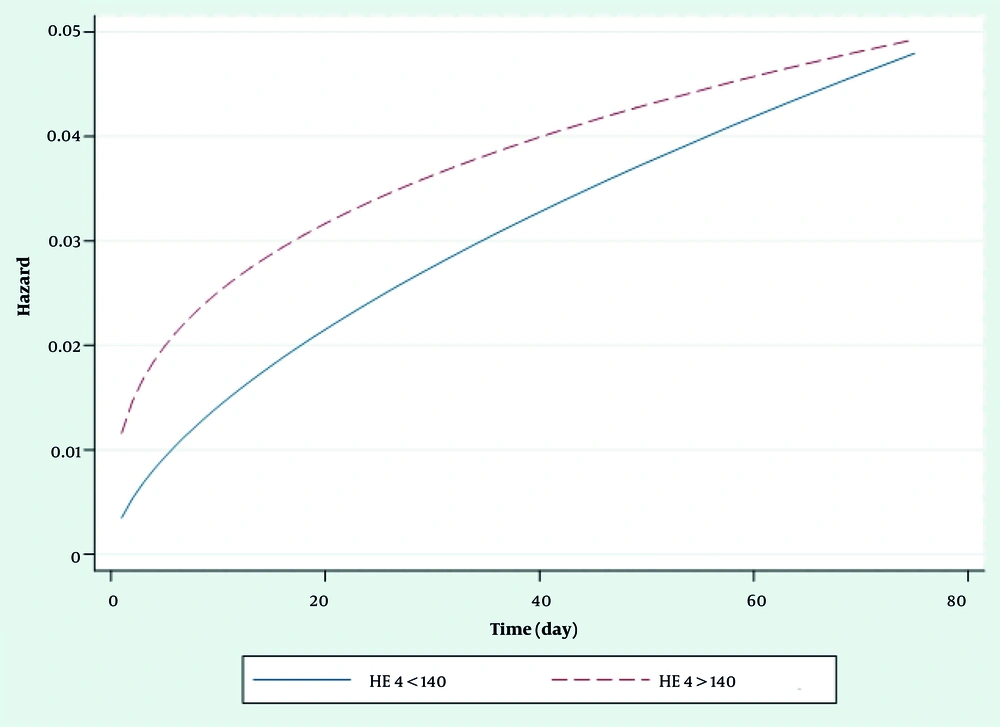
The shape of the hazard function for ovarian patients revealed an increasing trend, according to having HE4 > 140 or HE4 < 140 in EC (Figure 1).
5. Discussion
Our results confirm an association between high HE4 levels and other factors in EC in univariate and multivariate analyses.
The results of this study showed that the average age of the patients was 47.68 years in the age range of 35 - 67 years. The average ages of the patients in EC and non-EC groups were 49.48 and 45.47 years, respectively. The ages of menopausal and non-menopausal women averaged 55.36 and 40 years, respectively.
Several studies showed similar findings (17-19). For example, Liu et al. investigated serum HE4 levels in patients with EC. In their study, the average ages of the patient and control groups were 51 and 54 years, respectively (17).
In this study, the mean serum level of HE4 in menopausal and non-menopausal patients of the EC group was significantly higher than that of the non-EC group. Moreover, this serum level in menopausal patients of the EC group was significantly higher than that of non-menopausal women in this group. In a study by Abdalla et al. (20), the mean serum levels of HE4 in the case and control groups were 97.25 and 41.80 p mol.L, respectively. These amounts were 115.03 and 78.01, respectively, in our study. In the study of Jafari-Shobeiri et al. who evaluated the HE4 serum level before surgery in patients diagnosed with EC, 40 patients were compared with 60 healthy individuals. The cut point of HE4 was considered less than 70 pmol/L. The results of the study showed that the mean serum level of HE4 in EC patients was significantly higher than in healthy people (P < 0.01) (21).
In a study on the diagnosis of EC recurrence by serum HE4 in patients under routine clinical care, Donal et al. followed up 98 patients who were treated in an Australian medical center between 1999 and 2009 and observed relapse in 26 cases. The results of the study showed that with the start of the treatment, the serum level of HE4 decreased significantly (P = 0.001) and increased again with the recurrence of the disease (P = 0.002). The HE4 serum level increased by above 70 in 21 out of 26 patients (81%) (22).
In the study by Ge et al., a total of 254 patients with abnormal vaginal bleeding or discharge were included in the study. They used several candidate markers, including HE4. A new risk index for EC screening was developed by binary logistic regression. The most valuable diagnostic index for EC was HE4, followed by D-dimer and then fibrinogen (area under the receiver operating characteristic curve: Human epididymal protein 4 ¼ 0.794, D-dimer ¼ 0.717, fibrinogen ¼ 0.690) (22). The new risk index was superior to a single application of markers and a widely used combination (HE4 and CA125) (23).
The results showed that the serum levels of HE4 in all patients and menopausal non-menopausal patients with myometrial involvement were higher than those of patients without myometrial involvement. Antonseb et al. reported that the serum level of HE4 had a direct and significant relationship with the grading of patients and the degree of myometrial involvement (23). Zamani et al. observed a direct and significant relationship between the level of HE4 and tumor grading, the degree of visceral involvement, and the average size of the tumor caused by EC (24).
Regarding the entire cohort, in the univariate analysis of HE4, age and the history of infertility were able to discriminate prognosis for EC. In the multivariate model, HE4 displays a clear advantage in prognostic information in comparison with stage and age, exhibiting a hazard ratio of 1.89 for EC.
5.1. Conclusions
The overall findings indicate that serum HE4 protein levels increase with age and are significantly higher in patients with EC compared to control subjects, highlighting its potential as a biomarker for EC diagnosis. Additionally, HE4 serum levels show a direct and significant correlation with tumor grade and size, suggesting its usefulness in assessing the extent and severity of EC.
5.2. Limitation
Considering the cost of measuring HE4 serum levels and measuring this biomarker in limited patients, the statistical population of this research was limited, and to overcome the limitation, sampling was done accurately and without losing samples.