1. Background
Malignant ureteric obstruction might be due to primary malignancy of genito-urinary organs, such as the cervix, vagina, prostate, and bladder cancer, or non-urological origin, such as rectal or colonic carcinoma or retroperitoneal malignancies (1). The most common pelvic malignancy to cause extrinsic compression is carcinoma of the cervix in females and prostatic carcinoma in males (2). The onset of hydroureteronephrosis (HDUN) in a patient with malignancy, especially a pelvic malignancy such as carcinoma of the cervix, portends an advanced stage of malignancy either in terms of local infiltration or metastatic lymph nodal enlargement and poor prognosis (3). Obstructive anuria due to bilateral ureteric compression or infiltration has also been noted to be one of the presentations in a subset of patients with locally advanced pelvic malignancies (4, 5). Even though prostatic carcinoma is a frequently encountered malignancy, only a small proportion of patients with advanced and metastatic prostate carcinoma present with ureteral obstruction, requiring intervention (6). Currently, the involvement of a multi-disciplinary team has become a cornerstone in managing and treating patients with any malignancy. The oncological treatment with chemotherapy might be hindered as a result of renal dysfunction caused by ureteral obstruction.
Ureteric obstruction secondary to pelvic malignancies (UOPM) is managed by achieving urinary drainage either by internally relieving the obstruction [retrograde or antegrade double J (DJ) stenting] or by creating an external urinary diversion [percutaneous nephrostomy (PCN)] (7). A significant decrease in the quality of life is noted in patients who have undergone PCN compared to indwelling DJ stents (8). The recovery of renal function after relief of obstruction depends on the status of the renal parenchyma and renal cortical thickness at the time of DJ stenting or PCN (9). Timely diagnosis and efficient relief of UOPM is crucial in increasing renal failure-free patient survival and retaining the possibility of administering adjuvant chemotherapy.
2. Objectives
The aim of this study was to identify the factors influencing the feasibility of retrograde DJ stenting, antegrade DJ stenting, and PCN in patients with UOPM.
3. Methods
A retrospective analysis of all patients who underwent retrograde DJ stenting, antegrade DJ stenting, or PCN placement for UOPM over the past two years (January 2022 to December 2023) was done in our institute. Patients with incomplete data and chronic kidney disease were excluded from the study. Patients were divided into three groups based on the intervention type: Group 1: Retrograde DJ stenting, group 2: Antegrade DJ stenting, and group 3: PCN placement. All patients with HDUN on radiological imaging were initially given an attempt of retrograde DJ stenting under fluoroscopic guidance, failing which antegrade DJ stenting was attempted. Polyurethane DJ stent of size 5-French and 26 cm length was used for all patients who underwent retrograde or antegrade DJ stenting. PCN was inserted in case of failure to place a DJ stent in the ureter. A Malecot catheter of 12-French was used for PCN. Demographic, clinical, radiological, cystoscopic, and laboratory data were compared between the three groups. Ultrasonography was used to grade hydroureteronephrosis (HDUN) according to the Society for Fetal Urology (SFU) grading (10). In our study, SFU Grade 1 was referred to as mild, grade 2 as moderate, and Grades 3 and 4 as severe. Radiological imaging [Contrast Enhanced Computed Tomography (CECT) or Magnetic Resonance Imaging (MRI) of abdomen and pelvis for staging, extrinsic ureteric compression by tumor or metastatic lymph node, ureteric/trigonal infiltration by tumor], and bladder infiltration on cystoscopy were also compared between the three groups. The type of tumor, modality of management of primary malignancy (radiotherapy, chemotherapy, or surgery), presence of metastatic disease, and local recurrence after treatment were also considered in the study. Creatinine clearance ratio 1 (CCR1) was calculated by serum creatinine before the procedure – serum creatinine on a post-op day 1 × 100/ serum creatinine before the procedure, and albumin creatinine ratio (ACR) was calculated by preoperative serum albumin/ serum creatinine.
3.1. Statistical Analysis
Continuous variables were expressed as mean ± standard deviation, whereas categorical variables were presented as frequency and percentages. One-way ANOVA (Analysis of Variance) was used to assess the significance of various factors influencing the feasibility of retrograde DJ stenting versus antegrade DJ stenting versus PCN insertion. Multinomial logistic regression with univariate and multivariate analysis of the variables was used to analyze the relationship between the factors found to be significant on ANOVA and the type of intervention performed. The odds ratio was calculated for the variables with a confidence interval of 95%. The probability of the type of intervention with respect to the various factors was depicted using a marginal means plot. The statistical analyses were performed using Jamovi 2.3.28 software, and statistical significance was established by considering a P-value of less than 0.05.
4. Results
Fifty-nine patients with UOPM were included in the study, consisting of 14 males and 45 females, and the mean age of the study population was 60.1 ± 10.8 years. The clinicopathological characteristics of the study population are shown in Table 1. Out of the 59 patients, successful DJ stenting was achieved in 35 (59.3%) patients, whereas antegrade DJ stenting was carried out in 6 (10.2%), and 18 (30.5%) proceeded to PCN insertion.
| Variables | Retrograde Double J Stenting (n = 35) | Antegrade Double J Stenting (n = 6) | Percutaneous Nephrostomy (n = 18) | P-Value |
|---|---|---|---|---|
| Age | 59.1 ± 10.6 | 56.2 ± 12.9 | 63.2 ± 10.3 | 0.33 |
| Gender | 0.19 | |||
| Male | 6 (10.2) | 7 (11.9) | 1 (1.7) | |
| Female | 29 (49.2) | 11 (18.6) | 5 (8.5) | |
| Carcinoma cervix (n = 38) | 24 (63.1) | 3 (7.9) | 11 (29) | 0.64 |
| Carcinoma bladder (n = 7) | 3 (42.85) | 1 (14.3) | 3 (42.85) | 0.64 |
| Carcinoma prostate (n = 5) | 3 (60) | 0 | 2 (40) | 0.70 |
| Carcinoma colon (n = 3) | 2 (66.67) | 1 (33.33) | 0 | 0.27 |
| Carcinoma rectum (n = 3) | 1 (33.33) | 0 | 2 (66.67) | 0.36 |
| Carcinoma vagina (n = 2) | 1 (50) | 1 (50) | 0 | 0.14 |
| Carcinoma ovary (n = 1) | 1 (100) | 0 | 0 | 0.71 |
| Serum albumin | 3.59 ± 0.52 | 3.4 ± 0.66 | 3.05 ± 0.69 | 0.04 |
| Serum globulin | 3.36 ± 0.53 | 3.45 ± 0.84 | 3.41 ± 0.37 | 0.88 |
| Albumin globulin ratio | 1.09 ± 0.22 | 1.04 ± 1.03 | 0.90 ± 0.24 | 0.06 |
| Pre-operative serum creatinine | 2.64 ± 2.50 | 6.2 ± 2.63 | 3.92 ± 2.75 | 0.02 |
| Albumin creatinine ratio | 2.78 ± 2.27 | 0.62 ± 0.24 | 1.38 ± 1.20 | < 0.001 |
| CCR 1 | 12.33 ± 22.16 | 37.67 ± 25.92 | 12 ± 27.67 | 0.12 |
| Chemotherapy | 0.51 | |||
| Yes (n = 25) | 17 (28.8) | 2 (3.4) | 6 (10.2) | |
| No (n = 34) | 18 (30.5) | 4 (6.8) | 12 (20.3) | |
| Radiotherapy | 0.75 | |||
| Yes (n = 33) | 21 (35.6) | 3 (5.1) | 9 (15.3) | |
| No (n = 26) | 14 (23.7) | 3 (5.1) | 9 (15.3) | |
| Metastatic disease | 0.87 | |||
| Yes (n = 14) | 9 (15.3) | 1 (1.7) | 4 (6.8) | |
| No (n = 45) | 26 (44.1) | 5 (8.5) | 14 (23.7) | |
| Local recurrence | 0.47 | |||
| Yes (n = 13) | 9 (15.3) | 2 (3.4) | 2 (3.4) | |
| No (n = 46) | 26 (44.1) | 4 (6.8) | 16 (27.1) | |
| Hydroureteronephrosis | 0.005 | |||
| Mild/Moderate | 29 (49.2) | 4 (6.8) | 7 (11.9) | |
| Severe | 6 (10.1) | 2 (3.4) | 11 (18.6) | |
| Hydroureteronephrosis | 0.37 | |||
| Unilateral (n = 13) | 9 (15.3) | 2 (3.4) | 2 (3.4) | |
| Bilateral (n = 46) | 26 (44.1) | 4 (6.8) | 16 (27.1) | |
| On Radiological Imaging | ||||
| Extrinsic ureteric compression by tumor | 0.49 | |||
| Yes (n = 8) | 6 (10.1) | 1 (1.7) | 1 (1.7) | |
| No (n = 51) | 29 (49.2) | 5 (8.5) | 17 (28.8) | |
| Extrinsic ureteric compression by metastatic lymph nodes | 0.71 | |||
| Yes (n = 1) | 1 (1.7) | 0 | 0 | |
| No (n = 58) | 34 (57.6) | 6 (10.1) | 18 (30.6) | |
| Ureteric/bladder infiltration by tumor | 0.28 | |||
| Yes (n = 49) | 27 (45.8) | 5 (8.5) | 17 (28.8) | |
| No (n = 10) | 8 (13.5) | 1 (1.7) | 1 (1.7) | |
| Bladder trigonal infiltration on cystoscopy | 0.54 | |||
| Yes (n = 33) | 18 (30.6) | 3 (5.1) | 12 (20.3) | |
| No (n = 26) | 17 (28.8) | 3 (5.1) | 6 (10.1) | |
a Values are expressed as mean ± SD or No. (%).
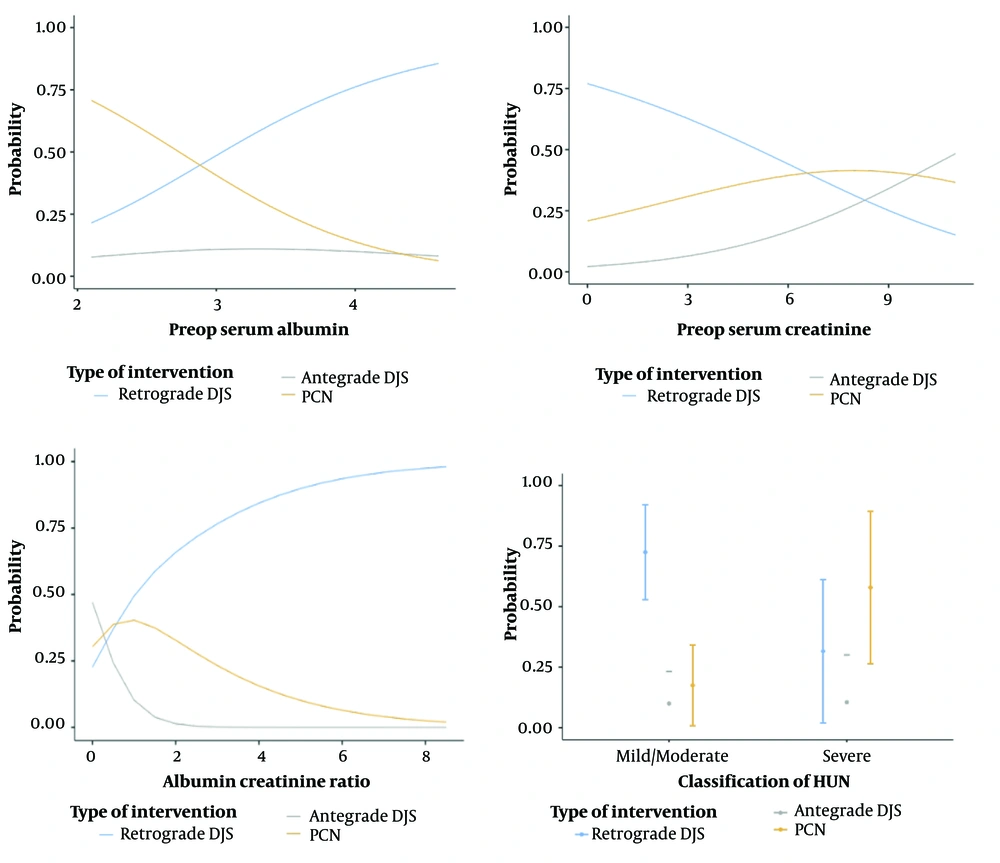
The mean age of the patients was similar between the retrograde DJ stenting, antegrade DJ stenting, and PCN groups (59.1 ± 10.6 vs. 56.2 ± 12.9 vs. 63.2 ± 10.3 years, P = 0.33). Of 59 patients, 49 (83%) had radiological imaging suggestive of ureteric or bladder infiltration, and 33 (55.9%) had bladder infiltration documented on cystoscopy. One-way ANOVA revealed statistical significance among the various groups of the following factors - Serum albumin F (2,12.7) = 3.88 (P = 0.04), serum creatinine F (2,13.5) = 5.05 (P = 0.02), ACR F (2,33.9) = 16.65, (P < 0.001), severity of HDUN (P = 0.013). No statistical significance was found when comparing CCR 1, the type of pelvic malignancy, prior completed treatment for malignancy such as chemotherapy, radiotherapy or surgical management, bladder infiltration on cystoscopic examination, and radiological ureteric or bladder infiltration with the type of intervention. All patients included in the study were found to have either locally advanced or metastatic disease.
A multinomial logistic regression analysis with separate univariate and multivariate analysis was conducted to determine the influence of serum albumin, serum creatinine, albumin creatinine ratio, and grade of HDUN on the type of intervention performed (Table 2). Univariate analysis between the PCN and retrograde DJ stenting groups revealed serum albumin (P = 0.006), ACR (P = 0.033), and severity of HDUN (0.002) to be significant. Comparison of antegrade DJ stenting and retrograde DJ stenting groups by univariate analysis revealed serum creatinine (P = 0.008) to be significant. Multivariate analysis revealed the severity of HDUN between PCN and retrograde DJ stenting group to be statistically significant (P = 0.02). The overall model in multivariate logistic regression was significant χ² (8) = 28.2 (P < 0.001). Estimated marginal means were calculated along with the plots for each variable, as shown in Figure 1, which reveal that the lower serum albumin, ACR value, higher serum creatinine value, and increasing severity of HDUN were associated with a lower probability of retrograde DJ stenting compared to antegrade DJ stenting or PCN placement.
| Comparison Groups and Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-Value | OR (95% CI) | P-Value | |
| PCN-retrograde DJS | ||||
| Serum albumin | 0.21 (0.07 - 0.65) | 0.006 a | 0.25 (0.07 - 0.93) | 0.05 |
| Serum creatinine | 1.22 (0.97 - 1.53) | 0.088 | 1.18 (0.80 - 1.73) | 0.39 |
| Albumin creatinine ratio | 0.60 (0.38 - 0.96) | 0.03 a | 0.88 (0.46 - 1.67) | 0.71 |
| HDUN; mild/moderate - gross | 0.13 (0.03 - 0.47) | 0.002 a | 0.16 (0.03 - 0.76) | 0.02 a |
| Antegrade DJS - retrograde DJS | ||||
| Serum albumin | 0.58 (0.13 - 2.53) | 0.47 | 2.04 (0.14 - 24.6) | 0.60 |
| Serum creatinine | 1.53 (1.12 - 2.10) | 0.008 a | 0.75 (0.23 - 2.37) | 0.62 |
| Albumin creatinine ratio | 0.09 (0.007 - 1.28) | 0.07 | 0.005 (1.82 × 10-7 - 189.6) | 0.33 |
| HDUN; mild/moderate - gross | 0.41 (0.06 - 2.78) | 0.36 | 0.32 (0.02 - 3.70) | 0.36 |
| PCN-antegrade DJS | ||||
| Serum albumin | 0.37 (0.07 - 1.83) | 0.22 | 0.15 (0.009 - 2.29) | 0.17 |
| Serum creatinine | 0.79 (0.59 - 1.07) | 0.13 | 1.57 ( 0.5 - 4.93) | 0.44 |
| Albumin creatinine ratio | 6.11 (0.47 - 78.8) | 0.16 | 150.8 (0.004 - 4.84 × 106) | 0.34 |
| HDUN; mild/moderate - gross | 0.32 (0.04 - 2.22) | 0.25 | 0.51 ( 0.05 - 5.51) | 0.59 |
Abbreviation: CI, confidence interval.
a P-value less than 0.5 was considered as significant.
5. Discussion
Our study compared the factors that affect the feasibility of different interventions for UOPM. We found the conversion rate from ureteric stenting to PCN to be 30.5%, similar to the study by Eshumani et al. (10, 11), where the mean conversion rate was 22.5%.
The type of primary malignancy has an impact when deciding to attempt either retrograde DJ stenting or antegrade stenting and PCN. Carcinomas arising from the prostate, bladder, and rectum have a higher chance of failure of DJ stent insertion due to their proximity and propensity for local infiltration of trigone and ureter compared to other pelvic and gastrointestinal malignancies (12). As per our study, the incidence of PCN insertion is higher among the patients with carcinoma of the rectum (66.67%), bladder (42.85%), and prostate (40%), respectively, in that order. Primary carcinoma of the vagina is very rare and, in the majority of cases, has ureteric obstruction either due to the malignancy itself or an associated uterine prolapse (13). Ovarian carcinoma causes ureteric obstruction by compression by mass effect or metastatic lesions rather than direct infiltration (14). Disease recurrence was noted in patients with HDUN with a history of surgical resection for colorectal carcinoma in our study, as observed in the study by Brown et al. (15).
Decreased serum albumin level is widely associated with an advanced stage of malignancy and poor prognosis (16, 17). Other factors, such as bladder invasion by primary tumor and reduced eGFR or raised serum creatinine, have also decreased the feasibility of successful retrograde DJ stenting and the need for PCN (18-20). Our study also revealed a significant decrease in serum albumin and an increase in serum creatinine among patients who had an unsuccessful attempt at retrograde DJ stent placement, which ultimately led to PCN insertion. In addition, when comparing the retrograde and the antegrade DJ stenting groups, the study revealed higher serum creatinine levels in the latter group and other compared variables being indifferent between the two groups.
The more severe HDUN is, the greater the chances of retrograde DJ stenting failure (21). This finding was also observed in our study, where in the probability of PCN placement increased with the severity of HDUN. Though we have not assessed the post-intervention resolution of HDUN and also long-term serum creatinine trajectory, studies have shown that PCN achieves a better resolution of HDUN and that the duration till baseline serum creatinine might be prolonged due to the higher serum creatinine levels in patients requiring PCN (22).
In our study, we used Polyurethane DJ stent of size 5-French and 26 cm length for all patients who underwent DJ stenting. The higher incidence of indwelling DJ stent failure in cases of malignant ureteral obstruction necessitates the conversion to PCN even though it has its disadvantages, such as prolonged hospital stays, incidences of accidental tube dislodgement, and impaired quality of life compared to DJ stent groups (10, 18, 19). However, metallic stents of various compositions have been introduced to tackle the issue of stent failure with the advantage of resisting luminal occlusion and an increase in stent change interval compared to standard Polyurethane DJ stent (22).
5.1. Limitations
We acknowledge that the study has limitations. It is a retrospective, single-center study with a limited sample size for various types of pelvic malignancies. Additionally, long-term follow-up data was unavailable, and the quality of life between different interventional groups was not assessed.
5.2. Conclusions
In conclusion, our study highlights the factors influencing interventions for UOPM. Though retrograde DJ stenting remained a common approach, a significant proportion of patients required PCN insertion. Serum albumin, serum creatinine, ACR, and severity of HDUN play crucial roles in determining the success of various interventions. Awareness of the likelihood of a successful intervention is beneficial when counseling the patient about the reality of the situation and what to expect during an attempt to manage UOPM. Further research with larger sample sizes and long-term follow-up data is warranted to validate these findings and optimize the management strategies for patients with UOPM.