1. Background
Bladder cancer is among the most common cancers worldwide, with an estimated incidence of over 500,000 new cases and 200,000 deaths in 2019 (1-3). Non-muscle invasive bladder cancer (NMIBC) accounts for approximately 75% of bladder cancer cases and is known for its high recurrence (50 - 70% within 5 years) and progression rates (10 - 30%) (4). Management strategies, including transurethral resection of bladder tumor (TURBT) and intravesical therapies aim at reducing recurrence and progression risks (3, 5). However, TURBT, with or without intravesical therapy, fails in about 50% of patients, increasing the likelihood of progression to muscle-invasive bladder cancer (MIBC) (6, 7). Factors such as lymphovascular invasion and high-grade tumors in re-TURB have been identified as predictors of response to bacillus Calmette-Guérin (BCG) treatment (8). Inflammation has been increasingly studied in cancer prognosis (9-11), with conflicting evidence regarding the impact of pyuria on NMIBC recurrence and progression (12-14).
2. Objectives
Given the importance of predicting BCG treatment response, this study aimed at evaluating bacteriuria and pyuria as potential predictors of intravesical recurrence (IVR) in NMIBC patients before BCG therapy.
3. Methods
3.1. Study Subjects
This study was conducted as a prospective cohort, enrolling consecutive patients referred to our institution. The patients included in the study had a primary urothelial bladder tumor and underwent TURBT. Additionally, they were candidates for intravesical BCG treatment within 2 to 4 weeks after surgery. The pathology results confirmed that the patients had NMIBC. Patients with variant histology, those with symptomatic bacteriuria requiring treatment, and individuals for whom BCG instillation was contraindicated were excluded from enrollment.
3.2. Patient Grouping
The participants enrolled in the research were categorized into 2 groups, depending on whether they exhibited pyuria/bacteriuria or not. Tumor recurrence was assessed at 3-month intervals during 3 years of follow-up. Before the initial administration of the BCG, a urine specimen was collected from each patient for microscopic analysis and culture. All participants in the study provided written agreement, and the research protocol received approval from the institutional review board (IR.TUMS.VCR.REC.1398.738).
3.3. Data Collection
A questionnaire was utilized to gather demographic and clinical data from the patients. The examined variables encompassed presence of pyuria, bacteriuria, proteinuria, hematuria, concurrent presence of carcinoma in situ (CIS), tumor grade, and recurrence of disease during follow up (36 months).
3.4. Urine Analysis
A urine analysis was conducted on the sample provided, using a catheter and immediately before the initial BCG instillation. Pyuria was defined as the presence of > 5 white blood cells/HPF in centrifuged urine sediment. Bacteriuria was characterized according to the widely accepted criteria described by Nicolle, (15) as the presence of ≥ 105 CFU/ml of urine with a single organism, indicating significant bacteriuria.
3.5. Data Analysis
A univariate analysis was conducted to examine the prognostic factors associated with IVR. Subsequently, multivariate analyses were conducted on the factors that exhibited a P-value below 0.2 in the univariate analysis. Finally, a subgroup univariable analysis was conducted on the variables under investigation. In our analysis, diabetes, CIS, and proteinuria were considered as potential confounders, and proteinuria was evaluated as an effect modifier in the association between pyuria and IVR. Moreover, there were no missing data for the analyzed variables. All patient records were complete, ensuring the integrity of our dataset.
4. Results
A total of 73 patients were included in the study. The mean (SD) age was 61.92 (12.27) years, and 15 (20.5%) patients were female. Pyuria was preoperatively observed in 31 (42.5%) patients. The relationship between pre-instillation pyuria and other clinicopathological variables is shown in Table 1. Pre-instillation pyuria was significantly associated with pre-instillation proteinuria (P < 0.001), pre-instillation hematuria (P = 0.023), pre-instillation bacteriuria (P = 0.001), and progression to MIBC (P = 0.028). Of the 73 patients, 24 (32.9%) developed IVR during a median follow-up of 36 months.
| Variables | Total | Preoperative Pyuria | P-Value | |
|---|---|---|---|---|
| Yes | No | |||
| Number of patients | 73 | 31 (42.5) | 42 (57.5) | |
| Age | 61.92 ± 12.27 | 63.81 ± 9.94 | 60.52 ± 13.69 | 0.262 |
| Gender | ||||
| Male | 58 (79.5) | 24 | 34 | 0.712 |
| Female | 15 (20.5) | 7 | 8 | |
| Smoke | 0.247 | |||
| Yes | 39 (53.4) | 19 | 20 | |
| No | 34 (46.6) | 12 | 22 | |
| Alcohol | 0.058 | |||
| Yes | 10 (13.7) | 7 | 3 | |
| No | 63 (86.3) | 24 | 39 | |
| Diabetes | 0.763 | |||
| Yes | 8 (11) | 3 | 5 | |
| No | 65 (89) | 28 | 37 | |
| Proteinuria | < 0.001 b | |||
| Yes | 32 (43.8) | 22 | 10 | |
| No | 41 (56.2) | 9 | 32 | |
| Hematuria | 0.023 b | |||
| Yes | 29 (39.7) | 17 | 12 | |
| No | 44 (60.3) | 14 | 30 | |
| Bacteriuria | 0.001 b | |||
| Yes | 53 (72.6) | 29 | 24 | |
| No | 20 (27.4) | 2 | 18 | |
| Primary tumor pathological grade | 0.428 | |||
| High | 20 (27.4) | 7 | 13 | |
| Low | 53 (72.6) | 24 | 29 | |
| CIS | 0.637 | |||
| Yes | 6 (8.2) | 2 | 4 | |
| No | 67 (91.8) | 29 | 38 | |
| MI IVR | 0.028 b | |||
| Yes | 6 (8.2) | 0 | 6 | |
| No | 67 (91.8) | 31 | 36 | |
| LPI (during 3 years (36 months) follow up | 0.347 | |||
| Yes | 13 (17.8) | 4 | 9 | |
| No | 60 (82.2) | 27 | 33 | |
| IVR | 0.362 | |||
| Yes | 24 (32.9) | 12 | 12 | |
| No | 49 (67.1) | 19 | 30 | |
Abbreviations: CIS, carcinoma in situ; MI, muscle invasion; LPI, lamina propria invasion; IVR, intravesical recurrence.
a Values are expressed as No. (%) or mean ± SD.
b P < 0.05 was considered statistically significant.
We examined the prognostic factors for postoperative bladder recurrence in all 73 patients. On univariable analysis, variables significantly associated with IVR were smoking, diabetes, concomitant CIS, and age (Table 2). A multivariable analysis revealed that diabetes (HR = 18.11, P = 0.004) and concomitant CIS (HR = 14.69, P = 0.039) were independent positive risk factors for IVR, while increasing age was an independent negative prognostic factor (HR = 0.938, P = 0.022).
| Variables | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-Value a | HR (95% CI) | P-Value b | |
| Gender | ||||
| Female vs. male | 0.44 (0.11 - 1.74) | 0.242 | ||
| Smoking | ||||
| Yes vs. no | 2.26 (0.82 - 6.25) | 0.116 | 1.40 (0.43 - 4.61) | 0.577 |
| Alcohol | ||||
| Yes vs. no | 0.86 (0.2 - 3.65) | 0.835 | ||
| Diabetes | ||||
| Yes vs. no | 7.83 (1.44 - 42.45) | 0.017 | 18.11 (2.53 - 129.8) | 0.004 |
| Proteinuria | ||||
| Yes vs. no | 1.45 (0.54 - 3.87) | 0.74 | ||
| Hematuria | ||||
| Yes vs. no | 1.13 (0.42 - 3.05) | 0.813 | ||
| Pyuria | ||||
| Yes vs. no | 1.58 (0.59 - 4.23) | 0.363 | 1.85 (0.51 - 6.78) | 0.352 |
| Bacteriuria | ||||
| Yes vs. no | 1.68 (0.53 - 5.33) | 0.382 | ||
| Grade | ||||
| High vs. low | 1.54 (0.53 - 4.49) | 0.428 | ||
| CIS | ||||
| Yes vs. no | 4.7 (0.80 - 27.76) | 0.088 | 14.62 (1.14 - 187.4) | 0.039 |
| MI | ||||
| Yes vs. no | 2.19 (0.41 - 11.77) | 0.361 | ||
| LPI | ||||
| Yes vs. no | 1.35 (0.39 - 4.67) | 0.637 | ||
| Age (y) | 0.97 (0.932 - 1.01) | 0.146 | 0.938 (0.89 - 0.99) | 0.022 |
Abbreviations: MI, muscle invasion; CIS, carcinoma in situ; OR, odds ratio.
a P ≤ 0.01 was considered statistically significant.
b P < 0.001 was considered statistically significant.
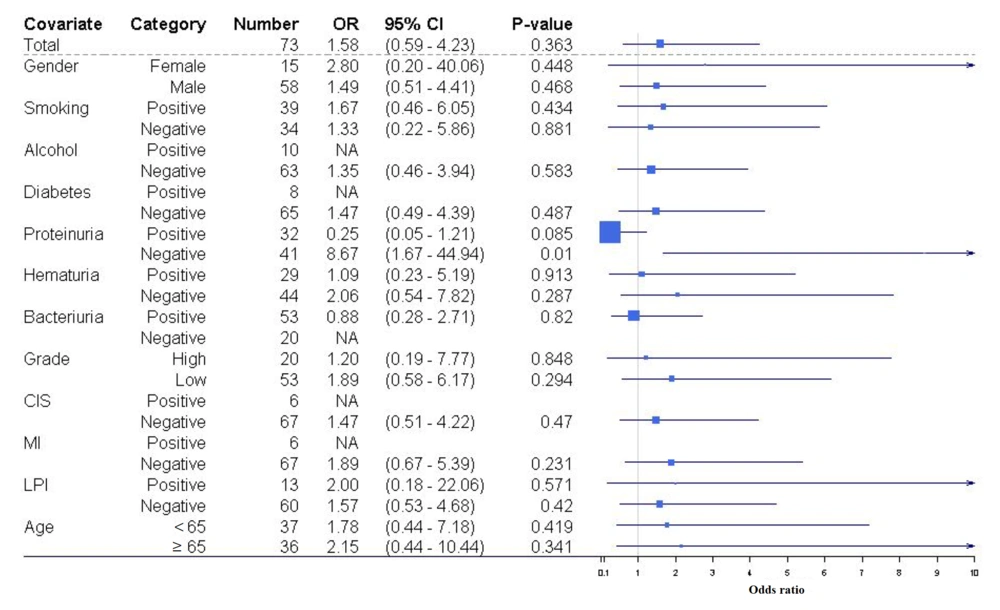
Given the limited sample size of patients (n = 73), subgroup univariable analysis was performed to evaluate the correlation between pyuria and IVR across various subgroups (Figure 1). We observed that pyuria emerged as a significant predictor of IVR only within a subset of patients devoid of proteinuria [odds ratio (OR) = 8.67, 95% CI = 1.67 - 44.9, P = 0.01].
Subgroup univariable analysis of the association between intravesical recurrence (IVR) and pyuria among different patient subgroups. The figure depicts analyses examining the correlation between IVR and pyuria across distinct patient subsets. Odds ratios (ORs) less than 1 signify a negative prognostic factor.
5. Discussion
In this study, we observed a 32.9% incidence of IVR among NMIBC patients undergoing BCG therapy. Our multivariate analysis identified younger age, diabetes, and CIS as independent risk factors for recurrence, while no significant association was found between pyuria and IVR in the overall population. However, subgroup analysis revealed a significant relationship between pyuria and IVR among patients without proteinuria, suggesting a potential interaction between inflammatory and metabolic factors in bladder cancer recurrence. These findings contribute to the ongoing efforts to refine risk stratification and optimize patient management in NMIBC.
Various studies have explored the relationship between aging and its influence on bladder cancer recurrence, yielding conflicting results. While some suggest a positive correlation, others find a negative association or no significant relationship between age and tumor recurrence (16-20). Joudi et al. observed reduced response to intravesical immunotherapy among older patients, while another study reported higher 5-year recurrence-free survival (RFS) rates in older patients (20). Yuge et al., in a study involving 400 patients with NMIBC, concluded that advanced age does not impact disease recurrence in those undergoing BCG therapy; instead, tumor characteristics determine treatment outcomes (16). Elderly individuals often experience declines in immune function, which may affect responses to treatments like BCG immunotherapy (21). Contrary to expectations, our study found that younger age elevates the risk of IVR. Given the varied literature findings and the influence of tumor grade and stage on bladder tumor recurrence, it is plausible that younger patients in our cohort had higher-grade tumors, potentially contributing to increased recurrence rates. Further research with larger datasets and molecular profiling is needed to clarify these discrepancies.
Our findings reinforce the strong association between diabetes and bladder cancer recurrence. Previous investigations have revealed a substantial correlation between diabetes and worse prognosis in several types of cancer, such as colon, breast, endometrial, liver, pancreatic, and bladder malignancies (21-25). However, the exact cause and method of this effect is not known. Potential mechanisms include chronic hyperglycemia, insulin resistance, and activation of insulin-like growth factor (IGF) pathways, all of which promote tumor proliferation and inhibit apoptosis. Moreover, diabetes-induced immune dysregulation could also impair the effectiveness of BCG therapy, reducing its anti-tumor activity (26-29). Given these findings, our study underscores the need for strict glycemic control in diabetic patients undergoing BCG therapy as a potential strategy to reduce recurrence risk. Multiple previous investigations have documented a significantly elevated risk of recurrence of NMIBC in individuals with diabetes compared to those without diabetes, with recurrence rates ranging from 60% to 45% in the former group and 40% to 30% in the latter group (30-32). The findings from a meta-analysis of 21 cohort studies comprising 13 million individuals indicated a noteworthy correlation between diabetes and a heightened risk of bladder cancer or cancer-related mortality (33). Our study's results align with these observations.
Carcinoma in situ is known to be a biologically aggressive entity with a high propensity for recurrence and progression, even in patients receiving adequate BCG therapy. Our findings also unveiled that CIS was a positive risk factor for IVR. This outcome is in line with previous studies that have documented a correlation between CIS and adverse outcomes concerning RFS and disease prognosis in bladder cancer patients (34-36). This highlights the importance of early detection and intensive monitoring of CIS-positive patients, as well as exploring potential combination therapies to enhance treatment efficacy.
In the realm of cancer development, pyuria has been studied as a potential marker of chronic inflammation and a risk factor for bladder tumor recurrence (37). Malignant tumors frequently exhibit substantial leukocyte infiltration, correlating with poorer prognoses (38-41). Notably, some studies have identified pyuria as a significant predictor for the ineffectiveness of BCG treatment (42). The simplicity and affordability of pyuria detection position it as an appealing option for biomarker analysis, providing economic advantages in cancer management (42). Multiple studies have associated pyuria with more aggressive forms of bladder cancer, characterized by deeper invasion and extensive mucosal lesions (12, 13, 43-45). Conversely, certain studies suggest that pyuria is not correlated with bladder cancer recurrence and survival (46). The variability in study outcomes may stem from various factors contributing to pyuria. For example, in a cohort study of individuals with NMIBC, negative urine cultures for bacteria alongside pyuria suggested localized inflammation associated with cancer (12).
While our overall analysis did not find a significant association between pyuria and IVR, a subgroup analysis was conducted to explore whether the association between pyuria and IVR varied across different clinical contexts. While this analysis was hypothesis-driven and based on known pathophysiological mechanisms, we acknowledge that subgroup analyses should be interpreted with caution due to the potential for overinterpretation and multiple comparisons. In our present study, pyuria was associated with IVR only in a subgroup of patients without proteinuria. This could be explained by the fact that proteinuria is linked to cancer-related inflammation and poorer prognosis; however, in the absence of proteinuria, pyuria may correlate with the severity of cancer-associated inflammation and higher likelihood of cancer recurrence.
Proteinuria has been explored as a potential biomarker in NMIBC, with some studies suggesting that it may predict worse RFS, possibly due to its association with systemic inflammation and endothelial dysfunction (47). However, our study did not find a significant correlation between proteinuria and IVR, which may be due to variability in the etiology of proteinuria in different patient populations. Prior research has highlighted that elevated urinary fibronectin levels correlate with increased recurrence risk, as fibronectin may inhibit BCG binding to the bladder wall (48). Despite these findings, the role of proteinuria in bladder cancer prognosis remains elusive, and further studies with molecular characterization are needed.
While smoking is a well-established risk factor for bladder cancer, its role in post-treatment recurrence remains debated. A recent meta-analysis, incorporating 15 studies with a substantial sample size of 10 192 patients, suggests that both current and former smoking are linked to an increased risk of recurrence and death in individuals diagnosed with bladder cancer (49). Additionally, a more recent systematic review and meta-analysis, involving 28 studies with 7 885 patients diagnosed with NMIBC, revealed that individuals with a history of smoking experience a less favorable prognosis in terms of RFS compared to non-smokers (50). However, our study did not find a significant association between smoking and IVR, which may be due to a low prevalence of smokers in our study or a lack of detailed smoking history (current vs. former smokers). Given the strong biological rationale linking smoking to tumor recurrence via DNA damage and chronic inflammation, future studies should incorporate detailed smoking categorizations and larger sample sizes to assess its true impact.
Our study found no statistically significant association between bacteriuria and IVR, which contrasts with previous findings suggesting that bacteriuria might decrease recurrence likelihood in individuals with NMIBC (14). Divergent findings across studies may stem from variations in operational definitions of urinary tract infections, potentially influencing (51).
Alongside the strengths and positive findings of our study, like all research, it has its limitations. One of the key limitations is the small sample size. However, we performed thorough statistical analyses to mitigate the impact of this limitation and ensure the reliability of our conclusions. Future studies with larger cohorts are needed to validate our findings further.
Although our findings demonstrated statistically significant associations, their clinical significance should also be considered. The association of diabetes and CIS with IVR aligns with previous studies and suggests that these factors may warrant closer monitoring in clinical practice. However, the lack of a significant association between pyuria and IVR in the overall population indicates that routine assessment of pyuria may not be a strong independent predictor of tumor recurrence in all patients. Further studies with larger sample sizes are needed to establish the clinical utility of these findings. In this regard, future research should focus on integrating clinical, metabolic, and molecular biomarkers to refine recurrence risk stratification in NMIBC. The observed interaction between pyuria and proteinuria in predicting IVR warrants further exploration, particularly through prospective multicenter trials and mechanistic studies examining inflammatory pathways. Moreover, incorporating liquid biopsy techniques and next-generation sequencing could provide deeper insights into tumor biology and treatment response.
5.1. Conclusions
Our study revealed that diabetes, CIS, and younger age were the only independent prognostic factors for IVR. Moreover, our study found no statistically significant association between pyuria, bacteriuria, and smoking with bladder tumor recurrence. However, our analyses demonstrated that pyuria emerged as a statistically significant predictive factor for IVR only among individuals without pre-instillation proteinuria.