1. Background
Sarcoidosis, a systemic illness characterized by non-caseating granulomas in various organs, primarily affects the lungs and intrathoracic lymph nodes (1). The exact cause of sarcoidosis remains unknown, but it is believed to stem from an exaggerated immune response triggered by environmental factors in genetically susceptible individuals (2). Several studies have examined the association between sarcoidosis and lung cancer, including a comprehensive investigation by Brincker and Wilbek involving 2,544 patients, which revealed a threefold higher incidence of lung cancer in sarcoidosis patients (3). However, the precise relationship between sarcoidosis and lung cancer remains elusive (4). Despite being relatively uncommon, the coexistence of sarcoidosis and lung cancer has been observed, particularly in cases of squamous cell lung malignancies, and has been associated with poorer survival rates (5, 6).
In recent years, lung cancer has emerged as the leading cause of cancer-related deaths worldwide, affecting both males and females, primarily due to its insidious onset, increased metastasis, and unfavorable prognosis (7-10). Lung cancer can be classified into two histological types: Small-cell lung carcinomas (SCLC) and non-small cell lung carcinomas (NSCLC), each with differing prognoses (11-13). Non-small cell lung carcinomas, the more prevalent and less aggressive form, accounts for approximately 88% of all lung cancer cases (14-16).
The discovery of microRNAs (miRNAs) and their regulatory role in gene expression has significantly advanced our understanding of cancer biology in the past decade (17-19). In cancer cells, certain miRNAs that inhibit genes involved in cell cycle progression and drive terminal differentiation are often downregulated, while others that regulate genes associated with cell cycle progression and resistance to apoptosis are overexpressed (20-22). In the context of inflammatory reactions, such as in sarcoidosis, miRNAs play a pivotal role in regulating the expression of key proteins that control the intensity of the innate and adaptive immune response (23-26). Multiple studies have demonstrated the dysregulation of various miRNAs in sarcoidosis.
2. Objectives
Considering the role of miRNAs in the pathogenesis of pulmonary diseases and their potential as diagnostic and prognostic tools, we aim at providing insights into the specific miRNAs that may contribute to possible associations between sarcoidosis and NSCLC. Therefore, we conducted quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) in the lymph nodes of patients with sarcoidosis and NSCLC as well as in the lymph nodes of controls to identify the differentially expressed miRNAs.
3. Methods
3.1. Subjects
For this study, we collected lymph node biopsy samples from patients with sarcoidosis NSCLC, and non-infectious lung diseases as the control group referred to Masih Daneshvari Hospital (n = 30 per group). The inclusion criteria of the study are to have symptoms of sarcoidosis as well as NSCLC and confirmation by a specialist doctor. Also, the exclusion criteria did not include any symptoms of sarcoidosis and NSCLC or other similar diseases that may interfere with the study results.
Also, all samples were prepared before any treatment such as chemotherapy or radiotherapy.
Radiographic, clinical, and histological features are used to diagnose sarcoidosis patients. Control lymph node samples were collected from patients without sarcoidosis and with other infectious diseases, who underwent mediastinoscopy or cervical lymph node biopsy during the same period. The research protocol received approval from the Clinical Ethics Committee of Masih Daneshvari Hospital (IR.SBMU.NRITLD.REC.1401.131).
3.2. RNA Extraction and RT-PCR
The expression of miR-192, miR-221, and miR-15 were detected by real-time RT–PCR. The RNA extraction phase was carried out, using the Cinna pure RNA, cat.No.PR891620 (SinaClon), according to the manufacturer’s instructions. The quality and quantity of the extracted RNA were evaluated, using NanoDrop spectrophotometery at 260 - 280 nm (27, 28). Subsequently, cDNA was synthesized from extracted RNA, using a reverse transcription system kit (Zist Royesh, Iran). Normalization was performed, using endogenous U6 RNA. The quantification of miRNAs was performed by real-time PCR according to the manufacturer’s protocol. The real-time PCR reactions were carried out, using the reverse transcription kit with specific miRNA primers. The expression of miRNAs was normalized to the mean of U6 RNA amounts, using the ∆∆Ct method.
3.3. Statistical Analysis
Statistical analysis was performed, using SPSS software version 20.0 (SPSS, Chicago, IL). Differences between the groups were examined for statistical significance by Two-sample binomial test. The relationship between the relative expression levels of the miRNAs was investigated by Spearman correlation coefficients. Receiver operating characteristic (ROC) curves and areas under the ROC curve (AUCs) were performed to assess the diagnostic ability of the miRNAs, and P < 0.05 was considered significant.
4. Results
There were no significant differences in the age between groups. The mean (SD) of age for sarcoidosis patients, NSCC patients, and control subjects was 46.58 (8.6), 47.2 (9.1), and 44.2 (8.3) years, respectively (P = 0.32).
4.1. Expression Levels of miR-192, miR-221, and miR-15
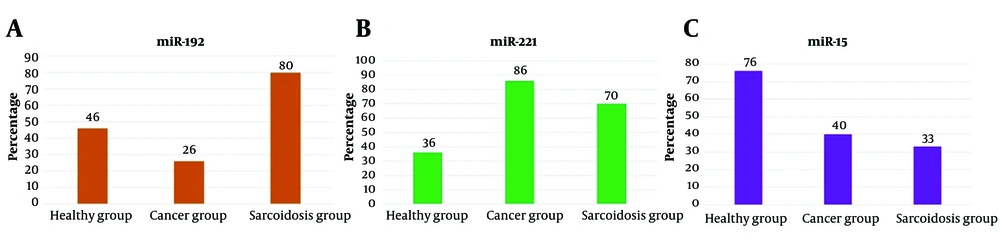
In lymph node samples from 30 sarcoidosis patients, the miR-192 biomarker tested positive in 24 individuals, while in NSCC tissue samples, it was positive in 8 out of 30 patients, and in healthy individuals, 14 out of 30 tested positive. Statistical comparison of the positivity rates revealed a significant difference (P < 0.001) (Figure 1A). miR-221 biomarker was detected in 21 out of 30 lymph node samples of patients with sarcoidosis, 26 out of 30 in NSCC patient samples, and 11 out of 30 in healthy individuals. The expression of this biomarker was significantly different between the study groups (P < 0.001) (Figure 1B). Furthermore, the miR-15 biomarker was detected in 10 out of 30 patients with sarcoidosis lymph node samples, 12 out of 30 in NSCC patient samples, and 23 out of 30 in healthy individuals. A statistical comparison of the positive rate of this biomarker indicated a statistically significant difference between the study groups (P < 0.001) (Figure 1C).
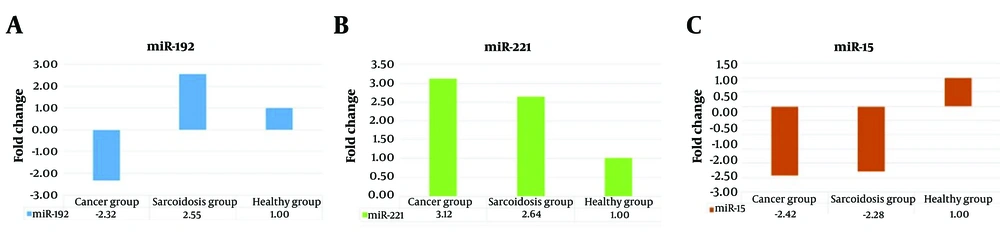
The results obtained using the ΔΔCT method showed that the relative expression of miR-192 was 2.55 times higher in patients with sarcoidosis compared to healthy individuals. In contrast, the relative expression of miR-192 was 2.32 times lower in NSCC tissue samples than in healthy individuals' samples (Figure 2A). This indicates a significant difference in the expression of miR-192 between the two groups, suggesting its potential as a biomarker for distinguishing between these conditions. Additionally, the relative expression of miR-221 in sarcoidosis and NSCC patients was 2.64 and 3.12 times higher than in healthy individuals, respectively. (Figure 2B). Furthermore, the relative expression of miR-15 in patients with sarcoidosis and NSCC tissue were 2.28 and 2.42 times lower than in healthy individuals, respectively (Figure 2C).
4.2. Correlation Between Expressed miRNAs
The relative expression levels of miR-192 were negatively correlated with miR-221 in sarcoidosis patients (r = -0.34, P < 0.05). There was a significant negative correlation between the relative expression of miR-221 and miR-15 (r = -0.41, P < 0.05). There was no significant correlation found between miR-192 and miR-15 in patients with sarcoidosis.
4.3. Evaluation of the Diagnostic Potential of miRNAs
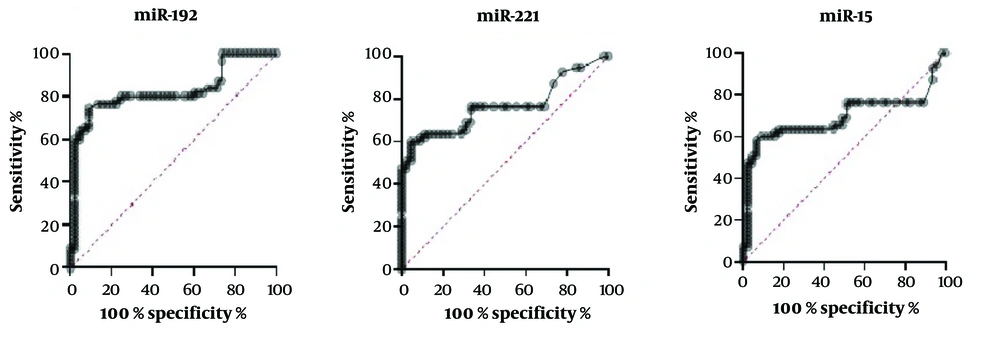
To investigate the characteristics of these miRNAs as potential diagnostic biomarkers of sarcoidosis, ROC curve analysis was performed on the miRNAs exhibiting significant differential expression. miR-192 showed the highest sensitivity of 82%, followed by miR-221 (71%), and miR-15 (69%) (Figure 3).
5. Discussion
Sarcoidosis, a systemic illness characterized by non-caseating granulomas in various organs, including the lungs, may present similarities with infectious, neoplastic, and granulomatous diseases. Additionally, evidence suggests that sarcoidosis may predispose individuals to lung cancer. Therefore, maintaining a diversified differential diagnosis and conducting a thorough assessment is crucial. In cases of sarcoidosis patients presenting with symptoms, lung cancer should be considered a potential differential diagnosis, whether due to causality or coincidence (29). In this study, 3 miRNAs were differentially expressed between patients with diseased individuals (sarcoidosis or NSCLC) and healthy controls, and there was a high similarity in miR-221 and miR-15 miRNA profiles between patients with sarcoidosis and those with NSCLC. Interestingly, miR-192 is differentially expressed between patients with sarcoidosis and NSCLC patients.
Numerous studies have investigated the expression of miRNAs in sarcoidosis and their potential influence on the pathogenesis of the disease. Several miRNAs, such as miR-15b, miR-27b, miR-192, miR-221, miR-222, miR-130a, and miR-let-7f are differentially expressed in peripheral blood mononuclear cells (PBMCs) of sarcoidosis patients compared to healthy controls. Additionally, miR-15, miR-192, and miR-221 have also shown differential expression in bronchoalveolar lavage (BAL) cells, which overlaps with their expression in PBMCs (30-32). Interestingly, miR-221 and miR-15 have shown high similarity in expression profiles between sarcoidosis patients and those with NSCLC (33).
Consistent with our study, previous studies demonstrated that miR-15 was significantly downregulated in NSCLC than in adjacent non-cancerous tissues. Its overexpression has been shown to inhibit cell viability, invasion, and migration while promoting apoptosis in NSCLC cells. Conversely, inhibition of miR-15 expression had the opposite effects on tumor progression. Studies have identified BCL2L2 as a target of miR-15 and demonstrated its negative regulation by miR-15a at the translational level. Thus, miR-15 acts as a tumor suppressor in NSCLC by directly targeting BCL2L2 and may serve as a potential diagnostic biomarker and therapeutic target (34). Furthermore, MiR-15a/miR-16 were frequently deleted or downregulated in squamous cell carcinomas and adenocarcinomas of the lung (35), suggesting that loss of this miRNA may be important in lung cancer development. However, there are conflicting results regarding the expression of miR-15 in sarcoidosis patients compared to healthy controls. Our results regarding miR-15 are not in line with those of Jazwa et al., who did not report any statistically significant differences between sarcoidosis patients and healthy controls about the expression of this miRNA (36). It seems that the downregulation of miR-15 in sarcoidosis patients, which we showed in our results, may contribute to the onset of lung cancer in these patients.
In the present study, interesting results were obtained in the case of miR-192, which showed significant upregulation in the lymph node of sarcoidosis. However, its expression was found to be significantly reduced in the lymph node of NSCLC patients. miR-192 is involved in angiogenesis and inflammation and has been reported to be upregulated in both BALF cells and PB lymphocytes of sarcoidosis patients. Further studies are needed to uncover the exact underlying mechanisms (32). Furthermore, miR-192 is downregulated in lung cancer tissues compared to adjacent lung tissue. Additionally, miR-192 is downregulated in the lungs of rats exposed to cigarette smoke and repressed in colon cancer (37, 38). miR-192, as a tumor suppressor, can inhibit the proliferation of lung cancer cells and promote their apoptosis by targeting the retinoblastoma 1 gene. The reduced expression of miR-192 in lung cancer samples suggests that it could be a potential target for therapeutic intervention in controlling the progression of cancer (39).
Our study also revealed increased expression of miR-221 in the lymph node cells of sarcoidosis and NSCLC patients. miR-221, along with miR-222, has been implicated in enhancing tumorigenic phenotypes, including invasiveness and resistance to apoptosis. These effects are achieved through the suppression of PTEN and TIMP3, both tumor suppressor genes (40, 41). The dysregulation of miRNAs, including those involved in inflammation and apoptosis, suggests the potential utility of miRNAs as diagnostic biomarkers in sarcoidosis (33).
The association between sarcoidosis and lung cancer has been studied extensively, with different theories proposed. One theory suggests that sarcoidosis precedes lung cancer and may even trigger malignant transformation due to postinflammatory scar tissue. Another theory proposes that sarcoidosis may develop as a consequence of lung cancer. Additionally, sarcoidosis has been considered an immune response to tumor antigens, as certain tumors can generate a systemic granulomatous response similar to sarcoidosis (42). However, the exact relationship between sarcoidosis and lung cancer remains unclear, and further research is needed to elucidate the underlying mechanisms.
5.1. Conclusions
This appears to be the first report of differentially expressed miRNAs in the lymph nodes of sarcoidosis and NSCLC. The results of this study indicate that the expression profiles of selected miRNA allow patients with sarcoidosis and NSCLC to be distinguished from potentially healthy controls. miR-221 and miR-15 in lymph nodes display similar expression patterns between patients with sarcoidosis and patients with NSCLC. However, miR-192 is differentially expressed in sarcoidosis and NSCC patients compared to the control group.
These miRNAs may have potential diagnostic and therapeutic implications, but further research is necessary to fully understand their roles in these diseases. Specifically, the identification of differentially expressed miRNAs needs to be validated in larger studies. The association between sarcoidosis and lung cancer is complex and requires more investigation to establish causality or coincidence between the two conditions and to identify possible disease biomarkers.