1. Background
Breast cancer (BC), a genetic disease, is characterized by inhibited cell death and uncontrolled cell proliferation (1). Current treatment methods for BC, such as chemotherapy, radiotherapy, surgery, and hormone therapy, are costly and come with significant side effects that often reduce quality of life and introduce other health-related complications (2, 3). The use of herbal compounds, especially antioxidants, may offer an effective alternative for the treatment and prevention of BC (4). Curcumin (CUR), a hydrophobic polyphenol derived from the root of the turmeric plant (Curcuma longa), is the active yellow pigment in turmeric and is known for its potential anti-cancer properties (5). Curcumin has demonstrated anti-tumor effects in several types of cancer, including colon, breast, and gastric cancers (6).
One of CUR’s crucial anti-cancer functions is inducing apoptosis by altering the expression of microRNAs (miRNAs) (7). miRNAs are small noncoding RNAs, typically 19 to 22 nucleotides in length, that regulate mRNA translation and stability by interacting with complementary sequences in the 3′-untranslated region (3′-UTR). miRNAs can target multiple genes and play roles in key biological processes, including homeostasis, angiogenesis, cell proliferation, differentiation, and apoptosis (8, 9). In BC, numerous miRNAs are dysregulated, including Mir-15a, which is particularly relevant due to its role in tumor cell survival, proliferation, and regulation of early cell differentiation (10). Studying these gene expression changes could provide new strategies for BC treatment, as functional studies have shown miRNAs to act as either oncogenes or tumor suppressor genes (11).
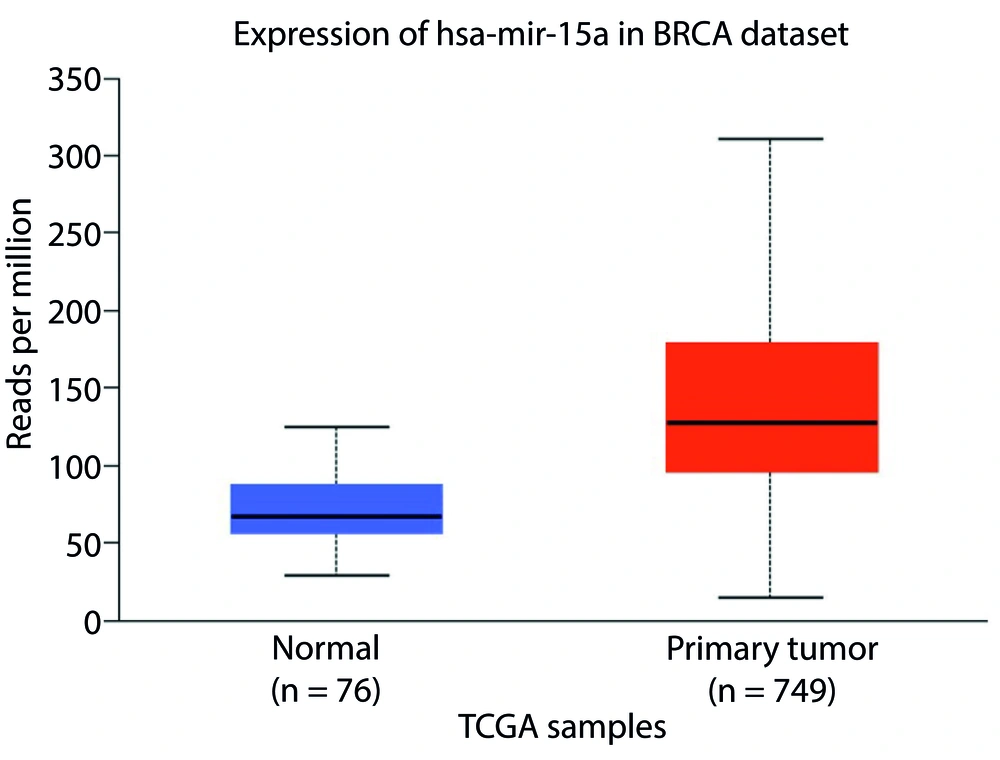
According to bioinformatics data, Mir-15a acts as an oncogene in BC, with approximately 70% increased expression (UALCAN database). Overexpressed oncogenic miRNAs in BC generally promote tumor growth by inhibiting tumor suppressor genes that regulate apoptosis (12). Research suggests that CUR has anti-cancer effects without notable toxicity and significantly inhibits the oncogenic activity of miRNAs. Thus, it is hypothesized that CUR reduces the oncogenic activity of Mir-15a while activating tumor suppressor genes, such as TP53, as a target gene (UALCAN database). The activation of tumor suppressor genes leads to changes in apoptosis pathway genes, specifically decreasing the level of the anti-apoptotic Bcl-2 protein (1, 13). Studies have shown that the interaction between anti-apoptotic Bcl-2 and pro-apoptotic Bax proteins is crucial in determining cell fate (12). We evaluated CUR’s inhibitory effects on MCF-7 cells and the role of Mir-15a in BC cell apoptosis upon CUR treatment. Our findings suggest that CUR significantly induces apoptosis in MCF-7 cells. Additionally, reductions in both Bcl-2 protein and Mir-15a levels are associated with CUR-induced apoptosis.
2. Objectives
This study assessed the effect of CUR at different concentrations (5 - 25 µM) on cell viability and the changes in Mir-15a and Bcl-2 expression levels through the induced apoptotic pathway in the MCF-7 cell line. The goal was to identify an effective and safe alternative treatment for BC, potentially surpassing the performance of traditional chemotherapy and radiotherapy.
3. Methods
3.1. Reagents
In this study, MCF-7 BC cells were obtained from the Pasteur Institute in Iran. These cells were cultured in RPMI1640 medium (Bio IDEA) supplemented with 1% Penstrap (Merck, Germany), 20% fetal bovine serum (FBS, Gibco, Invitrogen), and 3 mg/mL sodium bicarbonate (Serva Co., Germany). Curcumin and DMSO were provided by Sigma Aldrich, while EDTA (Bio IDEA), trypsin, and MTT (Solar Bio) were also used in the study. All materials required for the Real-time PCR analysis were sourced from Pishgam Company (Iran).
3.2. Cell Culture and Viability Studies
Human MCF-7 BC cells were obtained from the cell bank at the Pasteur Institute in Iran. Following cell culture, the cytotoxic effects of CUR on MCF-7 cells were assessed using the MTT assay at 24, 48, and 72 hours. Briefly, 10,000 cells were cultured in 96-well plates (NEST) and incubated in a 5% CO₂ environment at 37°C for 24 hours. The cells were then treated with varying concentrations of CUR (5 - 25 µM) at intervals of 24, 48, and 72 hours, with each concentration tested in triplicate. A control group was included, consisting of a triplicate set with zero µM drug dosage.
After the treatment periods of 24, 48, and 72 hours, the cell surface was isolated, and 10 µL of MTT (Solar Bio) solution along with 90 µL of fresh medium was added to each well, followed by a 4-hour incubation. Subsequently, the solution in each well was removed, and 100 µL of DMSO (Sigma Co., Germany) was added to dissolve the formazan crystals. Finally, the absorbance was measured using an ELISA reader (Elx808, BioTek, USA) at wavelengths ranging from 550 to 670 nm.
3.3. Total RNA Extraction and cDNA Synthesis
For RNA extraction, MCF-7 BC cells were seeded in two-well plates at a density of 10,000 cells per well. After 24 hours, the cells were treated with CUR and incubated for 48 hours. Subsequently, total RNA was extracted from both untreated and treated cells using Trizol (EasyBLUE total RNA extraction solution) according to the kit guidelines (Pishgam Biotechnology, Iran). The extracted RNA was stored in DEPC-treated water at -80°C. Complementary DNA (cDNA) synthesis was carried out using the RevertAid™ First Strand cDNA Synthesis Kit following the manufacturer's instructions. Primer design was conducted using GenRunner software, and the specificity of the designed primers was verified using NCBI software through a BLAST search against the genome. Table 1 presents the characteristics and specificity of the primers designed for Mir-15a, Bcl-2, B-actin, and U6 (Housekeeping Gene) genes.
| Gene | Forward (5 - 3) | Reveres (5 -3) |
|---|---|---|
| Bcl-2 | TAA CGG AGG CTG GGA TG | CAG GAG AAA TCA AAC AGA GGC |
| β-actin | CTT CCT TCC TGG GCA TG | GTC TTT GCG GAT GTC CAC |
| Mir-15a | CGGGCTAGCAGCACATAATG | CAGCCACAAAAGAGCACAAT |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
3.4. Real-time PCR
The real-time PCR SYBR Green protocol was developed by Pishgam Company (Iran) and conducted using the Rotor-Gene 6000 (Corbett Research, Australia). Each reaction included 1 μL of each primer, 7 μL of double-distilled water, 1 μL of cDNA (2 ng), and 10 μL of SYBR Green PCR Master Mix, resulting in a total volume of 20 μL. The thermal cycling conditions were set as follows: 15 seconds at 95°C, 20 seconds at 60°C, and 30 seconds at 72°C, repeated for 40 cycles.
3.5. Flow Cytometry
Flow Cytometry analysis was conducted using a BD Biosciences (USA) flow cytometer with two groups: MCF-7 cells cultured in media as controls and cells treated with CUR at the IC50 concentration for 48 hours. Two-well plates were prepared for this test, with 10,000 cells seeded in each well, followed by 24 hours of incubation. The cells were then treated with CUR (IC50 concentration) for 48 hours. After incubation, the cells were washed with PBS, trypsinized, and resuspended in PBS. The cell suspension was then mixed with Annexin-V (Biolegend) and incubated for 10 minutes. Data were collected and analyzed using Partec Flomax software.
3.6. Statistical Analysis
Data analysis was conducted using SPSS version 22. For real-time PCR data, relative expression was calculated using REST software (Germany). Gene expression and flow cytometry data were then analyzed using an unpaired t-test.
4. Results
4.1. MTT Assay
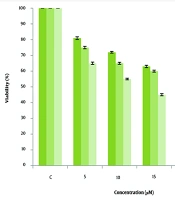
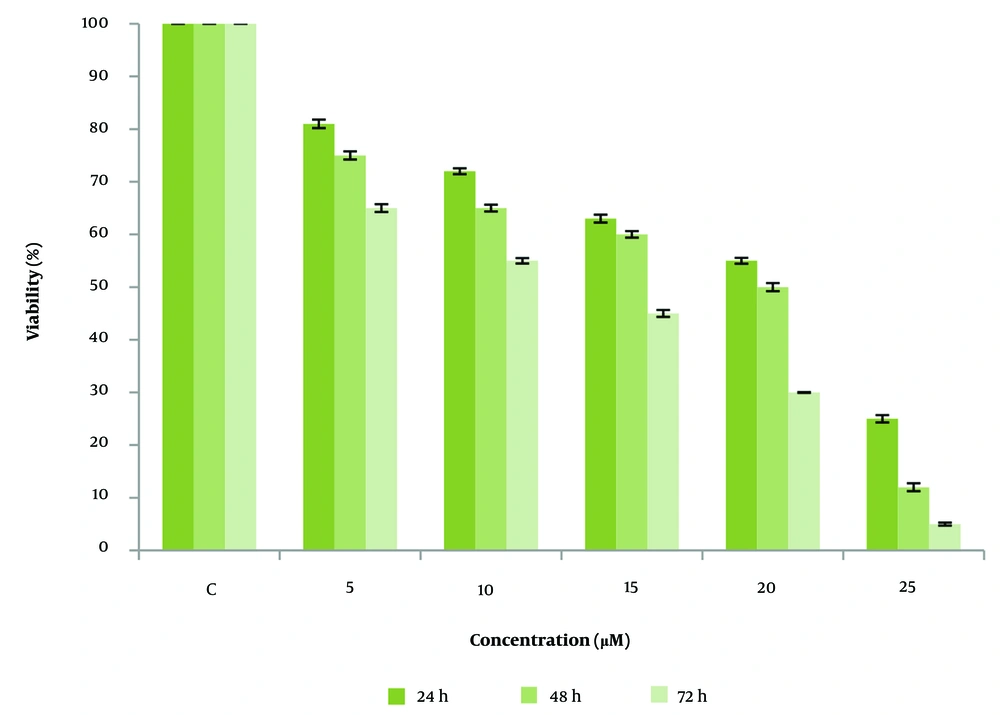
The MTT Assay results indicate that cell viability is concentration-dependent on CUR. Curcumin reduced cell viability in a dose-dependent manner, with a significant reduction observed after 72 hours of treatment at 25 µM, highlighting the compound's anti-cancer potential. Furthermore, the IC50 value for MCF-7 cells treated with CUR was determined to be 20 µM after 48 hours (Figure 1).
4.2. Flow Cytometry
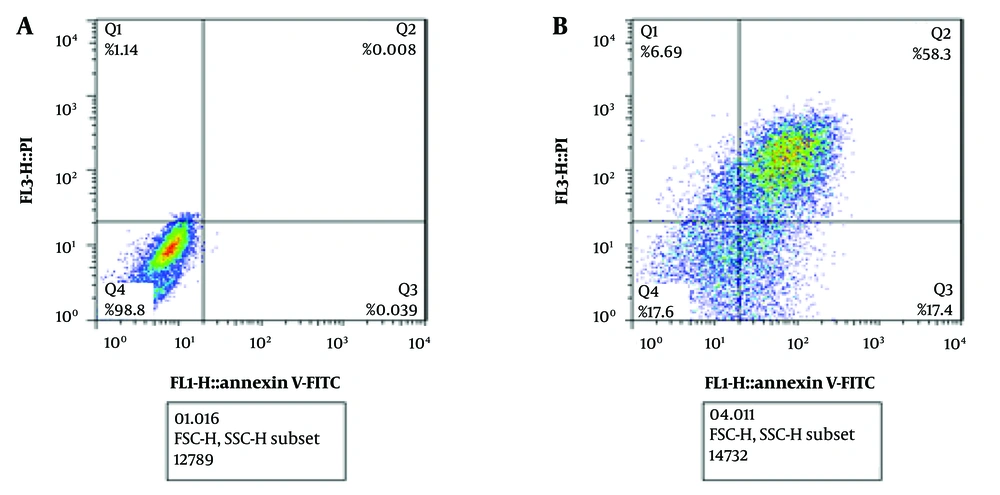
Flow Cytometry analysis was conducted to assess apoptosis induction by CUR. The results demonstrated that treating MCF-7 cells with the IC50 concentration (20 μM) of CUR for 48 hours resulted in 75.7% apoptosis, a 75% increase compared to the control group (Figure 2 and Table 2). These findings confirm that CUR effectively induces apoptosis and promotes cell death in MCF-7 cells.
| Test | Sample | |
|---|---|---|
| Untreated Cells | Cells Treated with Piperine at IC50 Concentration and 48 Hours | |
| Necrosis (%) | 1.14 | 6.69 |
| Late apoptosis (%) | 0.008 | 58.3 |
| Early apoptosis (%) | 0.039 | 17.4 |
| Live cells (%) | 98.8 | 17.6 |
| Unpaired t-test | Mean = 0.023 | Mean = 37.8 |
| Significant | P-value < 0.001 | |
4.3. Real-time PCR
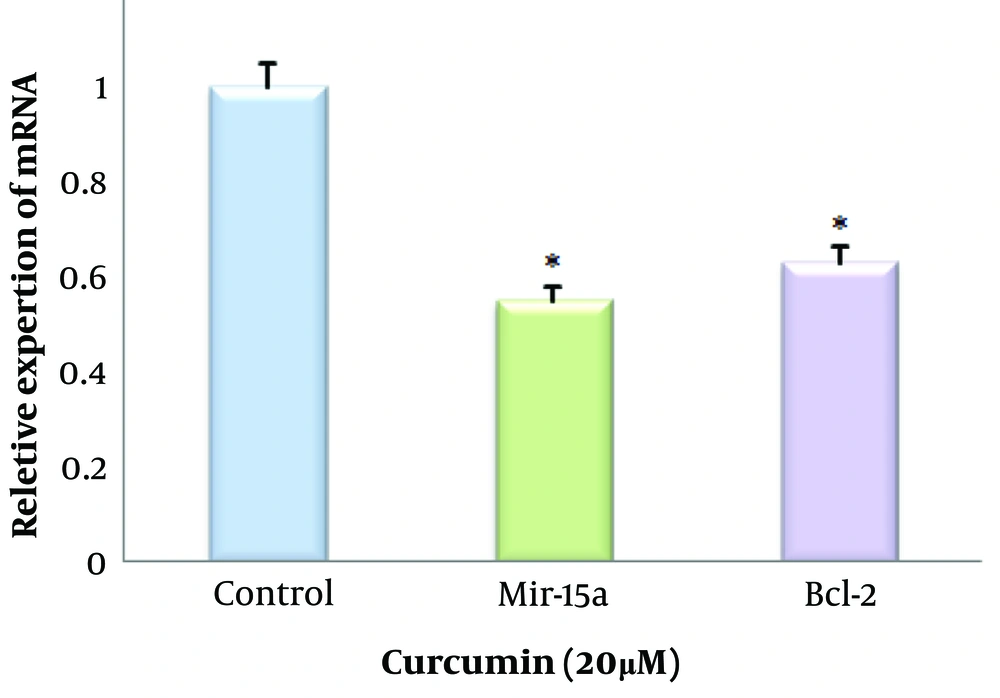
We used bioinformatics tools to analyze Mir-15a expression in BC cells (UALCAN site). Figure 3 illustrates the expression of Mir-15a in BC cells, regulated by CUR at an IC50 concentration (20 µM) in the MCF-7 cell line. A comparison of Mir-15a expression between control and CUR-treated cells revealed an approximate 0.55-fold decrease in expression in the treated cells. Additionally, Bcl-2 expression levels in the control and CUR-treated cells showed an approximate 0.63-fold reduction in the treated cells (Figure 4).
5. Discussion
Curcumin is a natural compound extracted from Curcuma longa (14). Traditionally used as a natural remedy, recent research has underscored its therapeutic potential, including antibacterial, anti-inflammatory, antiviral, anti-tumor, and anti-aging effects (15, 16). Studies indicate that CUR possesses anti-cancer properties and can induce apoptosis, thereby inhibiting cell proliferation in BC cell lines (17). Curcumin has also been shown to reduce HER-2 expression and inhibit cell proliferation in MCF-7 cells that overexpress HER-2 (18).
miR-15a has been reported to be overexpressed in several human tumors, including BC, where it exerts oncogenic activity by inhibiting tumor suppressor genes, leading to increased cell proliferation and inhibition of apoptosis. The regulation of cell proliferation depends on a balance between cell division and apoptosis. Apoptosis, or programmed cell death, is essential for this balance, and CUR has demonstrated the ability to induce apoptosis in various cancer cells (19, 20). In this study, flow cytometry analysis showed a significant increase in the number of early and late apoptotic BC cells following CUR treatment.
The Bcl-2 family members are crucial regulators of cell death and survival. An abnormally high expression of Bcl-2 protein promotes BC development. Bcl-2 and Bax together form an "apoptotic switch" in tumorigenesis and cancer treatment, positioning Bcl-2 family members as potential targets for cancer therapy (21).
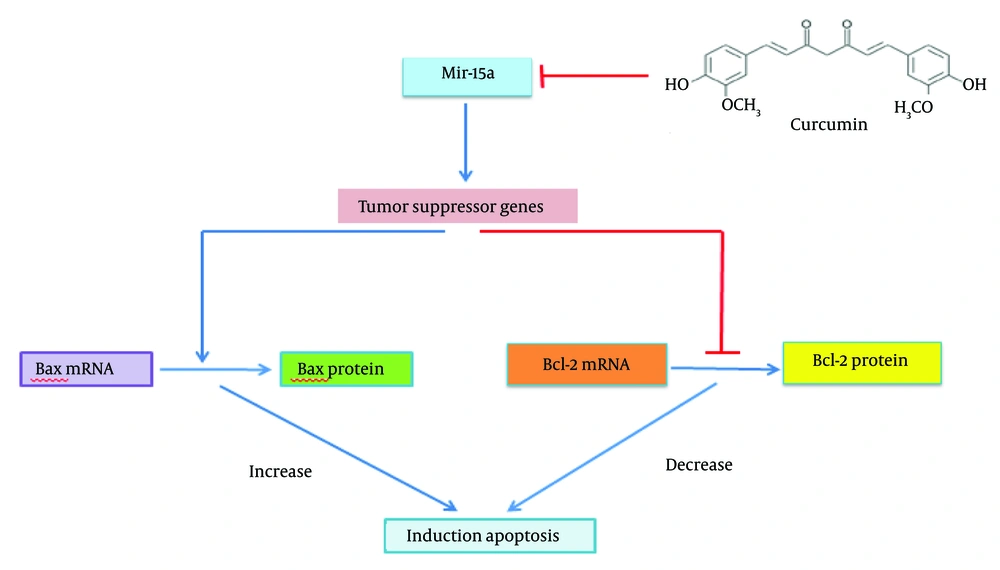
The activation of tumor suppressor genes is necessary to initiate the apoptosis pathway. CUR reduces the oncogenic activity of miR-15a, leading to the activation of tumor suppressor genes, which subsequently initiate apoptosis by downregulating Bcl-2 protein expression (Figure 5).
In a brief overview, Jiang et al. demonstrated that Aloe-emodin significantly impacts the proliferation of breast tumor (BT) cells by promoting apoptosis. Aloe-emodin induces apoptosis in BT cells by regulating miR-15a/miR-16-1 expression, which subsequently inhibits Bcl-2 (22). Additionally, Patel et al. observed that the overexpression of miR-16 and miR-15a downregulates BMI1, leading to mitochondria-mediated apoptosis (23). In Iran, Haghi et al. conducted experiments on human BC cell lines MDA-MB-231 and SK-BR-3, treating them separately or in combination with hsa-miR-34a-5p and hsa-miR-16-5p mimics. Their results revealed that both miR-16 and miR-34a induced apoptosis and cell cycle arrest, while also inhibiting migration and invasion in both cell lines (24). Similarly, Esmatabadi et al. found that Mir-21 inhibits various anti-cancer effects of dendrosomal curcumin (DNC) in BC cells. Their findings suggest that combining DNC treatment with the downregulation of Mir-21 could be a beneficial strategy for tackling drug-resistant BC cells (25).
In this study, we assessed the effect of CUR on MCF-7 cells, specifically focusing on Mir15-a and Bcl-2 gene expression. The MTT assay results indicated that treatment with CUR (5, 10, 15, 20, and 25 μM) for 24, 48, and 72 hours significantly inhibited the viability of MCF-7 cells compared to the control group. Flow cytometry results further showed that 75.7% of the cells underwent apoptosis at the IC50 concentration following 48 hours of treatment. Additionally, real-time PCR results revealed a decrease in the expression of mir-15a and Bcl-2 in MCF-7 cells at the IC50 concentration and 48-hour treatment period.
5.1. Conclusions
In brief, this study demonstrates that CUR can effectively inhibit BC progression by reducing the expression of Mir-15a, an oncogene, while activating tumor suppressor genes, such as TP53, a target of Mir-15a, and inducing apoptosis through Bcl-2 inhibition. Consequently, CUR holds promise as an effective agent for BC treatment within next-generation drug delivery systems.