1. Background
Vision disorders are one of the most common adverse reactions seen in patients with non-small cell lung cancer (NSCLC) (1). Indeed, low-grade visual disturbances were frequently reported across a variety of pivotal crizotinib clinical trials (2-6). Recently, an investigation among a patient subgroup (n = 33) enrolled in PROFILE 1001 found that 66.7% experienced at least one ophthalmologic change from baseline in some assessments following treatment with crizotinib; however, the majority of these ocular events with crizotinib treatment were of low-grade severity (7). Nonetheless, crizotinib has been associated with ocular events of high-grade severity, such as severe visual loss (SVL) (1).
A number of factors may predispose patients with NSCLC to develop SVL. First, patients with lung cancer are often treated with cytotoxic chemotherapy, which is associated with ocular toxicities (8). Second, approximately 20% of patients with lung cancer develop brain metastases (9, 10). Treatment modalities for brain metastases include radiotherapy and stereotactic brain surgery (11, 12), which can lead to complications such as optic neuropathy, retinopathy and cortical blindness (13-17). Finally, medical conditions, such as hypertension, diabetes, and macular degeneration, are prevalent in the elderly population, and these conditions may also predispose patients to SVL (18-22).
2. Objectives
We therefore conducted an observational investigation to assess the frequency of risk factors for and sequelae of SVLs and potential sight-threatening events (PSTEs) among patients treated with crizotinib to better understand low-grade PSTEs as well as high-grade SVLs.
3. Methods
3.1. Study Design
We conducted a non-interventional (NI), enhanced global pharmacovigilance (PV) study of adult patients treated with crizotinib from March 31, 2016, to March 31, 2021. This study collected data on adverse events (AEs) and severe adverse events (SAEs) indicative of SVL or PSTEs from ongoing/new crizotinib clinical trials (including both Pfizer-sponsored and non-Pfizer-sponsored trials), ongoing/new crizotinib NI primary data collection studies, post-marketing spontaneous reports, and other solicited sources (e.g., compassionate use programs). Data for this study were collected through the routine data collection practices of AE/SAE reporting from these data sources (i.e., through standard PV procedures), with enhanced data collection as described below. To be eligible, patients must have been treated with crizotinib and had at least one AE/SAE report indicative of SVL or PSTE received during the study period. All reports indicative of SVL or PSTE in patients who had been treated with crizotinib were included, regardless of the crizotinib indication for use. There were no exclusion criteria.
3.2. Variables
Cases indicative of SVL in all clinical trials were identified by grade 3 or grade 4 eye disorders based on common terminology criteria for adverse events (CTCAE) (23). According to CTCAE, grade 3 eye disorders include symptomatic retinopathy with marked decrease in visual acuity or disablement. Grade 4 eye disorders based on CTCAE are blindness in the affected eye. Cases indicative of SVL in NI primary data collection studies, spontaneous reports, and other solicited data sources were identified by the following preferred terms (PTs) (24) that correspond with grade 3 or grade 4 events on CTCAE: Blindness, blindness cortical, blindness day, blindness transient, blindness unilateral, amaurosis, amaurosis fugax, night blindness, sudden visual loss, optic neuropathy, optic ischemic neuropathy, optic nerve disorder, retinopathy, toxic optic neuropathy, visual cortex atrophy, visual pathway disorder, optic atrophy, hemianopia, hemianopia heteronymous, hemianopia homonymous, quadrantanopia, tunnel vision, and visual field defect. Cases indicative of PSTE in all Pfizer-sponsored clinical trials as well as non-Pfizer-sponsored clinical trials with a first subject, first visit (FSFV) that occurred after June 30, 2017, included all identified grade 2 eye disorders and other grade 2 eye disorders of retinal detachment, retinal edema, maculopathy, iritis, uveitis, and visual field tests abnormal. For non-Pfizer-sponsored crizotinib trials with a FSFV that occurred up to June 30, 2017, including NI primary data collection studies, spontaneous reports, and other solicited data sources, the following PTs were used for cases indicative of PSTE: Retinal detachment, retinal edema, maculopathy, iritis, uveitis, and visual field tests abnormal. Other relevant variables are described in Appendix 2 in the Supplementary File.
3.3. Data Collection
Study-specific follow-up questionnaires were created and provided to investigators in crizotinib clinical trials and crizotinib NI primary data collection studies; additionally, a data capture aid was created to assist Pfizer drug safety unit staff in their local countries to collect additional data on PSTEs/SVLs from certain crizotinib NI primary data collection studies, post-marketing spontaneous reports, other solicited data sources, and non-Pfizer-sponsored crizotinib clinical trials in which the FSFV occurred up to June 30, 2017. All questions on the follow-up questionnaire and the data capture aid were the same; two separate forms were used due to operational and logistical needs. An external adjudication committee, comprised of three experts in research and clinical ophthalmology, provided additional scientific integrity for the study by determining whether cases reported with AEs/SAEs potentially indicative of PSTE or SVL were likely to be true cases. The committee used a priori defined criteria in tandem with expert clinical judgment to adjudicate the reported events. Each case was independently reviewed by two members of the committee and classified for the endpoint of PSTE, SVL, non-case (i.e., neither SVL nor PSTE), or insufficient information for adjudication. In the case of disagreement, a third (blinded) adjudicator was brought in to serve as a tiebreaker. If disagreement remained, then the committee met to discuss the case until a majority classification was determined.
3.4. Statistical Methods
No statistical hypotheses or sample sizes were specified in the protocol or statistical analysis plan for this study; thus, no inferential statistical analyses were performed, and all analyses were descriptive in nature. Descriptive statistics were used for continuous variables [e.g., number of observations (n), mean, standard deviation, minimum, median, and maximum] and for categorical variables (e.g., counts and percentages). Missing values were not imputed; however, partial date values were imputed when possible. The frequency of risk factors and outcomes of SVL and PSTEs were analyzed at three different levels: Patient, event, and eye, as appropriate. Three different analysis levels were used because a patient may have had multiple events in one or both eyes. The patient level comprised all unique patients for whom one or multiple PSTEs or SVLs were reported. The event level was defined as all adjudicated PSTEs or SVLs reported as part of this study. Since there were no patients with multiple adjudicated events, the event level and the patient level were the same. The eye level was defined as the affected eye. For patients with only one eye affected, the contralateral eye was summarized as “unaffected eye”.
3.5. Protection of Human Patients
This study was based on data collected through routine AE/SAE reporting procedures, following standard PV practices, with enhanced data collection as previously described. As such, data were obtained through established reporting mechanisms rather than through direct patient interaction or intervention. Therefore, no study-specific consent form was required. Additionally, the information used in this study was anonymized before the study was conducted and analyzed retrospectively. As a result, institutional review board/independent ethics committee review and approval were not required. Nevertheless, this study was conducted in accordance with applicable legal and regulatory requirements and followed generally accepted research practices in pharmacoepidemiology (25). This study was also registered on the Heads of Medicines Agencies-European Medicines Agency catalogue of real-world data studies, which replaced the European Union electronic register of post-authorization studies (26), with the following registration number: EUPAS12963.
4. Results
4.1. Cohort Identification and Adjudication
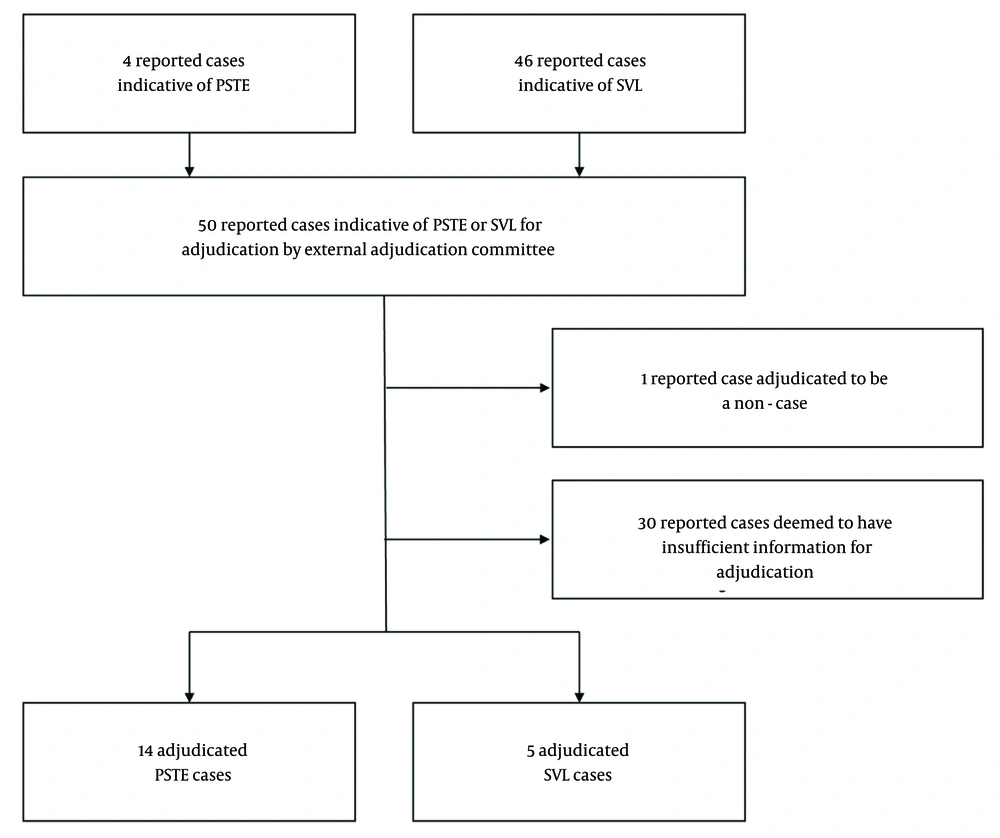
Overall, 50 cases indicative of SVL or PSTE were received during the study period from Africa, Asia, Europe, and North America via spontaneous reports (n = 42), Pfizer-sponsored clinical trials (n = 2), non-Pfizer-sponsored clinical trials (n = 5), and other solicited data sources (n = 1). Of these cases, 46 (92.0%) were reports potentially indicative of SVL, and 4 (8.0%) were reports potentially indicative of PSTE. Of the 46 cumulative reported cases potentially indicative of SVL, five (10.9%) cases were adjudicated as likely to be SVL, 10 (21.7%) cases as likely to be PSTE, one (2.2%) report was adjudicated to be a non-case, and there was insufficient information for the remaining 30 (65.2%) cases. Adjudication of the four reported cases indicative of PSTE found all four (100%) cases as likely to be PSTE. Therefore, in total, there were 14 adjudicated PSTE cases, five adjudicated SVL cases, one non-case, and 30 cases with insufficient information (Figure 1 and Table 1). Details on associated patient characteristics (e.g., age, race, geographic location, etc.) can be found in Appendices 1 and 3 in the Supplementary File.
| Variable | Reported Cases Indicative of PSTE or SVL | ||
|---|---|---|---|
| PSTE (N = 4) | SVL (N = 46) | Total (N = 50) | |
| Adjudicated outcome | |||
| PSTE | 4 (100.0) | 10 (21.7) | 14 (28.0) |
| SVL | 0 (0.0) | 5 (10.9) | 5 (10.0) |
| Insufficient information | 0 (0.0) | 30 (65.2) | 30 (60.0) |
| Non-case | 0 (0.0) | 1 (2.2) | 1 (2.0) |
| Total | 4 (100.0) | 46 (100.0) | 50 (100.0) |
Abbreviations: N, number of PSTE or SVL events; PSTE, potential sight-threatening event; SVL, severe visual loss.
a Values are expressed as No. (%).
b PSTE or SVL events where both eyes are affected are counted only once.
4.2. Severe Visual Loss/Potential Sight-Threatening Event Risk Factors
(1) Ocular history: Among the five adjudicated SVL cases, a history of ocular disease prior to the SVL occurrence was reported in four (80.0%) cases, including two cases of cataracts in the affected eye, one case of macular edema in the affected eye, one case of retinal holes in the unaffected eye, and one case of retinal detachment in the affected eye. Ocular history was not reported for the remaining SVL case. Among the 14 adjudicated PSTE cases, there was no history of any ocular disease prior to the PSTE occurrence for one (7.1%) case; for the remaining 13 (92.9%) PSTE cases, the history of ocular disease was unknown (Table 2).
| Variables | Adjudicated PSTE (N = 14) | Adjudicated SVL (N = 5) | Total (N = 19) |
|---|---|---|---|
| History of an ocular disease | |||
| Yes | 0 (0.0) | 4 (80.0) | 4 (21.1) |
| No | 1 (7.1) | 0 (0.0) | 1 (5.3) |
| Unknown/not reported | 13 (92.9) | 1 (20.0) | 14 (73.7) |
| Medical history | |||
| Diabetes | 1 (7.1) | 2 (40.0) | 3 (15.8) |
| Hypertension | 3 (21.4) | 1 (20.0) | 4 (21.1) |
| Hyperlipidemia | 0 (0.0) | 1 (20.0) | 1 (5.3) |
| Transient ischemic attack/stroke | 1 (7.1) | 0 (0.0) | 1 (5.3) |
| Elevated intracranial pressure | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Exposure to treatment with ocular toxicity | 1 (7.1) | 0 (0.0) | 1 (5.3) |
| Brain metastases | |||
| Yes | 2 (14.3) | 3 (60.0) | 5 (26.3) |
| No | 1 (7.1) | 1 (20.0) | 2 (10.5) |
| Unknown/not reported | 11 (78.6) | 1 (20.0) | 12 (63.2) |
| Ophthalmologic examinations prior to occurrence of PSTE or SVL | |||
| Yes | 1 (7.1) | 1 (20.0) | 2 (10.5) |
| No | 0 (0.0) | 2 (40.0) | 2 (10.5) |
| Unknown/not reported | 13 (92.9) | 2 (40.0) | 15 (78.9) |
Abbreviations: N, number of PSTE or SVL events; PSTE, potential sight-threatening event; SVL, severe visual loss.
a Values are expressed as No. (%).
b PSTE or SVL events where both eyes are affected are counted only once.
(2) Medical history and prior exposures: The medical history of the adjudicated SVL cases included diabetes (40.0%), hypertension (20.0%), and hyperlipidemia (20.0%). Additionally, no adjudicated cases of SVL had a prior history of exposure to medications associated with ocular toxicity. For the adjudicated PSTE cases, medical history included diabetes (7.1%), hypertension (21.4%), and transient ischemic attack/stroke (7.1%). Moreover, there was one (7.1%) adjudicated PSTE case in which the patient took a medication with potential ocular toxicity prior to the onset of the PSTE (i.e., binimetinib) (Table 2).
(3) Brain metastases: Three (60.0%) SVL cases were reported to have brain metastases prior to the occurrence of SVL; the brain metastases involved the optic nerve, visual pathway, or occipital lobe for one SVL case, did not involve the optic nerve, visual pathway, or occipital lobe for another SVL case, and involvement was unknown for the remaining SVL case. Additionally, two (14.3%) PSTE cases were reported to have brain metastases prior to a PSTE; involvement of the optic nerve, visual pathway, or occipital lobe was unknown for both PSTE cases (Table 2).
(4) Prior ophthalmologic examinations: Two of the five (40.0%) adjudicated SVL cases had no ophthalmologic examination, and one (20.0%) adjudicated SVL case had one ophthalmologic examination within one year prior to the start of crizotinib treatment. Information about ophthalmologic examinations within one year prior to the start of crizotinib was unknown for the remaining two (40.0%) cases. For the one SVL case who had an ophthalmologic examination, the subject had a history of an epiretinal membrane. Of the 14 adjudicated PSTE cases, one (7.1%) had an ophthalmologic examination within one year prior to the start of crizotinib treatment; associated information was unknown for the remaining 13 (92.8%) cases. For one one PSTE case, there were no significant findings in the ophthalmologic examination (Table 2).
4.3. Crizotinib Exposure
Crizotinib dosing information was reported for four of the five (80.0%) adjudicated SVL cases. The median time from the first exposure to crizotinib until the onset of a SVL event was 77 days (min, max: 41, 152). The median cumulative days of treatment with crizotinib prior to onset of SVL was 76.5 days (min, max: 41, 152). The median total daily dose of crizotinib prior to the onset of SVL was 500 mg (min, max: 250, 500). Crizotinib dosing information was reported for 9 (64.3%) of the 14 adjudicated PSTE cases. The median time from the first exposure to crizotinib until onset of a PSTE was 8 days (min, max: 1, 1068). The remaining dosing information was available for 7 (50.0%) of the 14 adjudicated PSTE cases. The median duration of exposure prior to onset of a PSTE was four days (min, max: 1, 671). The median total daily dose of crizotinib prior to the onset of a PSTE was 500 mg (min, max: 250, 500) (Table 3).
| Parameters | Adjudicated PSTE | Adjudicated SVL | Total |
|---|---|---|---|
| Time from first exposure to crizotinib until onset of the PSTE or SVL (d; N) | 9 | 4 | 13 |
| Mean ± SD | 241.3 ± 391.89 | 86.8 ± 51.99 | 193.8 ± 329.51 |
| Median (range) | 8.0 (1 - 1068) | 77.0 (41 - 152) | 41.0 (1 - 1068) |
| Number of cumulative days being treated with crizotinib prior to the onset of the PSTE or SVL (d; N) | 7 | 4 | 11 |
| Mean ± SD | 155.6 ± 271.57 | 86.5 ± 52.23 | 130.5 ± 215.14 |
| Median (range) | 4.0 (1 - 671) | 76.5 (41 - 152) | 41.0 (1 - 671) |
| Total daily dose of crizotinib immediately prior to onset of the PSTE or SVL (mg; N) | 7 | 4 | 11 |
| Mean ± SD | 392.9 ± 133.63 | 437.5 ± 125.00 | 409.1 ± 126.13 |
| Median (range) | 500.0 (250 - 500) | 500.0 (250 - 500) | 500.0 (250 - 500) |
| Average daily dose of crizotinib prior to the onset of the PSTE or SVL (mg; N) | 7 | 4 | 11 |
| Mean ± SD | 411.8 ± 118.55 | 437.5 ± 125.00 | 421.2 ± 115.27 |
| Median (range) | 500.0 (250 - 500) | 500.0 (250 - 500) | 500.0 (250 - 500) |
Abbreviations: N, number of PSTE or SVL events; PSTE, potential sight-threatening event; SD, standard deviation; SVL, severe visual loss.
a Time from first exposure until onset of PSTE or SVL is calculated as date of onset of PSTE or SVL - date of first exposure to crizotinib +1 day. The number of cumulative days being treated with crizotinib is defined as the sum of days the patient who experienced the PSTE or SVL has been treated with any dose of crizotinib between the treatment start date with crizotinib and the onset date of the PSTE or SVL event. The total daily dose immediately prior to the onset of the PSTE or SVL is defined as the total daily dose the patient who experienced a PSTE or SVL was taking the day before the onset of the PSTE or SVL. The average daily dose is defined as the cumulative dose of crizotinib prior to the onset of the PSTE or SVL divided by the cumulative number of days treated with crizotinib. The PSTE or SVL events where both eyes are affected are counted only once.
4.4. Outcomes of the Adjudicated Cases
All five (100%) adjudicated SVL cases were serious. As of the data cut-off date, four (80.0%) SVL cases were ongoing, and the status of one (20.0%) case was unknown. Two (40.0%) cases received treatment for SVL, one (20.0%) case did not receive treatment, and the treatment status was unknown for two (40.0%) cases. Two (40.0%) cases were reported as not related to crizotinib, and the relationship was unknown for three (60.0%) cases. Of the 14 adjudicated PSTE cases, 10 (71.4%) cases were serious, three (21.4%) were non-serious, and the seriousness was unknown for one (7.1%) case. Two (14.3%) cases received treatment for PSTE, one (7.1%) did not receive treatment, and the treatment status was unknown for 11 (78.6%) cases. Of the 10 serious cases, four (40.0%) were ongoing at the time of the data cut-off date, three (30.0%) resolved without sequelae, and three (30.0%) had an unknown outcome. Four PSTE cases were reported as not related to crizotinib and the relationship was unknown for 9 cases; there was one (7.1%) PSTE case considered related to crizotinib, and the status of this case was ongoing at the time of data cut-off (Table 4).
| Variables | Adjudicated PSTE | Adjudicated SVL | Total |
|---|---|---|---|
| Outcome (N) | 14 | 5 | 19 |
| Resolved without sequelae | 3 (21.4) | 0 | 3 (15.8) |
| Resolved with sequelae | 0 | 0 | 0 |
| Ongoing | 7 (50.0) | 4 (80.0) | 11 (57.9) |
| Unknown | 4 (28.6) | 1 (20.0) | 5 (26.3) |
| Outcome of serious events (N) | 10 | 5 | 15 |
| Resolved without sequelae | 3 (30.0) | 0 | 3 (20.0) |
| Resolved with sequelae | 0 | 0 | 0 |
| Ongoing | 4 (40.0) | 4 (80.0) | 8 (53.3) |
| Unknown | 3 (30.0) | 1 (20.0) | 4 (26.7) |
| Outcome of the PSTE or SVLs related to crizotinib (N)c | 1 | 0 | 1 |
| Resolved without sequelae | 0 | 0 | 0 |
| Resolved with sequelae | 0 | 0 | 0 |
| Ongoing | 1 (100.0) | 0 | 1 (100.0) |
| Unknown | 0 | 0 | 0 |
| Outcome of the serious PSTE or SVL, related to crizotinib (N) c | 0 | 0 | 0 |
| Resolved without sequelae | 0 | 0 | 0 |
| Resolved with sequelae | 0 | 0 | 0 |
| Ongoing | 0 | 0 | 0 |
| Unknown | 0 | 0 | 0 |
| Treatment of the PSTE or SVL | 14 | 5 | 19 |
| Yes | 2 (14.3) | 2 (40.0) | 4 (21.1) |
| No | 1 (7.1) | 1 (20.0) | 2 (10.5) |
| Unknown | 11 (78.6) | 2 (40.0) | 13 (68.4) |
Abbreviations: N, number of PSTE or SVL events; PSTE, potential sight-threatening event; SVL, severe visual loss.
a Values are expressed as No. (%) unless otherwise indicated.
b For the outcome of bilateral PSTE or SVLs, the worse outcome of both eyes is tabulated. The outcomes from best to worse are: Resolved - resolved with sequelae - ongoing. Bilateral PSTE or SVLs are counted only once in this table. Percentages are calculated based on the number of PSTE or SVL events or a subset of PSTE or SVL events as appropriate.
c Cases with an unknown relationship to crizotinib are not included in this section.
After the onset of SVL, two of the five adjudicated cases (40.0%) had slit lamp examination with no abnormalities found in the anterior chamber; one (20.0%) had no slit lamp examination performed, and the examination status was unknown for two (40.0%) cases. After the onset of PSTE, the status of slit lamp examinations was unknown for all 14 cases. Two of the five (40.0%) patients with an adjudicated SVL event and one (7.1%) with an adjudicated PSTE had a visual field test performed after their SVL/PSTE onset (data not shown).
5. Discussion
This enhanced PV study examined 50 cases indicative of SVL or PSTE received between March 31, 2016, and March 31, 2021, and adjudicated by an independent adjudication committee. Of these cases, five were adjudicated as likely to be SVL and 14 as likely to be PSTE; moreover, one case was adjudicated to be a non-case, and there was insufficient information for the remaining 30 cases. Associated risk factors for SVL/PSTE were noted among the adjudicated cases: Prior history of ocular disease, diabetes, hypertension, hyperlipidemia, and transient ischemic attack/stroke. In addition, 60.0% of the adjudicated SVL cases and 14.3% of the adjudicated PSTE cases had a prior history of brain metastases.
Although the nature of our investigation prevented the estimation of incidence, we observed that the proportion of adjudicated SVLs and PSTEs reported here (i.e., 26.3% and 73.7%, respectively) was consistent with previous clinical trials that indicated a higher occurrence of low-grade PSTEs compared to high-grade SVLs among crizotinib-treated patients (1-6). Despite the inability to directly compare to prior clinical trials, this study helps contextualize the current literature on the real-world safety profile of crizotinib. For example, a recent post-authorization safety study that used routinely collected health data in Denmark, Finland, Sweden, the Netherlands, and the United States from 2011 to 2017 found that vision disorders were generally underreported among crizotinib-treated patients; however, the authors stated that this trend was expected because most vision disorders are under-recorded in routinely collected healthcare data (27). Our study thus provides more descriptive data on these milder vision disorders that are under-recorded in certain real-world data sources.
Additionally, an analysis of ALK inhibitors conducted within the Food and Drug Administration (FDA) adverse event reporting system concluded that eye disorders were a significant safety signal among crizotinib-treated patients (28), thereby highlighting the significance of providing data on risk factors for PSTE/SVL to improve patient care. Indeed, vision loss and visual impairment can negatively affect quality of life, independence, mental health, social function, and educational attainment (29). Therefore, it is important for clinicians to take proactive steps to monitor visual symptoms among crizotinib-treated patients, as is recommended in the FDA label (1); this recommendation is especially critical in the context of crizotinib’s expanded indication for pediatric patients with anaplastic large cell lymphoma and inflammatory myofibroblastic tumor (30, 31). However, our results suggest that ophthalmological examinations may not be regularly conducted during routine care due to the lack of information received on ophthalmologic examinations performed prior to and after the occurrence of a PSTE/SVL.
Nonetheless, as an enhanced PV study that mainly relied on reported AE/SAE data, incomplete information for a large proportion of cases prohibited us from fully evaluating the reported cases. Similarly, high levels of missing data were also observed, thereby resulting in measurement error. These limitations were expected though as 84.0% of the reported cases were received from the spontaneous reporting system, which can be an inefficient tool for the surveillance of AEs (32-34). Notably, less detailed information on risk factors was recorded for PSTE cases compared to SVL cases, leaving us with even less insight into the PSTE cases overall. While the data did not provide explicit reasons for this discrepancy, we hypothesize that (spontaneous) reporters may have provided only minimal information for PSTEs because these events, by their nature, were less severe (compared to SVLs) and thus likely not viewed as requiring critical or detailed documentation.
Additional limitations of this study included the small sample size (i.e., only 19 reports adjudicated as true SVL or PSTE cases) and the absence of confounder control or a comparator group, which limited our ability to perform robust statistical analyses for causal inference. Moreover, the results of this study may not be generalizable to the broader NSCLC patient population taking crizotinib, in part due to the reliance on spontaneous reporting data, which may not fully represent the diversity of patients treated in real-world settings. Furthermore, the geographically dispersed nature of the patient cohort makes it challenging to apply conclusions to any single country or region.
Despite these limitations, a major strength of this study was the utilization of the enhanced PV study design. Given the rarity of both the target condition (35) and the safety outcome of interest, it would not have been feasible to employ conventional observational study designs, as they would have been hampered by enrollment and/or follow-up issues. Thus, the enhanced PV study design provided us an efficient approach to collect a large amount of important safety information on a sizable and geographically dispersed cohort of exposed patients.
Another strength was the inclusion of the adjudication committee to better classify the received reports. Adjudication committees have typically been used in clinical trial research (36, 37); however, they can add value in the context of PV investigations where there is a lack of standardized definitions for the endpoints of interest and/or when data quality is variable. The adjudication committee determined that 30 reported cases had insufficient information for evaluation and that one reported case was not a true SVL/PSTE. Therefore, the utilization of an adjudication committee reduced the effect of information bias on our results. Future researchers in this area should consider incorporating adjudication committees into their PV investigations when there are potential concerns over data quality; however, researchers must also be aware that this approach can result in a reduced sample size and should therefore integrate other approaches (e.g., inclusion of other data sources) to ensure an adequate sample size for analysis. Any future investigations in this area would also be strengthened by adding design elements aimed at exploring any potential biological mechanisms.
5.1. Conclusions
Upon evaluation of the independently adjudicated reports of SVL and PSTE in this enhanced PV study, a history of ocular diseases, pre-existing medical conditions, and the presence of brain metastases were identified in a large proportion of SVL cases; however, less information on these risk factors was recorded for the PSTE cases. Based on the overall assessment of the low number of cases, many of which had limited and/or missing information, no new vision safety signals associated with crizotinib were identified in this investigation.