1. Background
Kidney cancer incidence in India is rising, with an annual rate of 17,480 cases and a 5-year prevalence of 1.4 per 100,000 individuals (1). Renal cell carcinoma (RCC) constitutes about 70-85% of kidney tumors (2, 3). The RCC's high metastasis rate stems from angiogenesis and neovascularization, causing hematogenous spread, with larger tumors showing increased incidence (4). Common metastasis sites include lungs, bone, and brain, while adrenal glands, the opposite kidney, and liver may also be affected (5).
Metastatic RCC poses a significant health challenge with overall survival rates below 10% (6, 7). Currently, imaging modalities such as contrast enhanced computed tomography (CECT), positron emission tomography (PET), and magnetic resonance imaging (MRI) are employed for initial workups of metastasis in RCC patients. However, the subsequent monitoring of these patients becomes financially burdensome and logistically challenging due to the repeated use of these imaging techniques and the need for contrast administration and radiation exposure. There is a pressing need for novel and simple predictors of metastasis in patients with RCC to aid in early detection, thereby having prognostic and therapeutic implications.
High platelet counts and an increase in inflammatory mediators such as neutrophils and macrophages have been known to be associated with advanced stages of malignancies (8-10). In RCC, the role of various inflammatory markers like neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), Systemic Inflammatory Response Index (SIRI), and Systemic Immune-Inflammatory Index (SII) in prognostication has been studied in localized and locally advanced RCC extensively (11, 12). However, limited studies have evaluated the role of inflammatory marker ratios in metastatic RCC (13, 14). As per current literature, there is a paucity of data on the usefulness of SIRI, SII, NLR, and PLR in predicting metastasis in RCC (15).
2. Objectives
We performed this study to assess the predictive accuracy of NLR, PLR, SIRI, and SII in detecting metastases in patients with RCC.
3. Methods
A retrospective analysis was conducted at a tertiary care hospital, analysing a prospectively maintained patient database from January 2022 to December 2023. Institute ethics committee approval was obtained (IEC1: 214/2024).
All patients with age over 18 years diagnosed with RCC in our institute and whose hematological studies were conducted within one week before treatment were included in the study. The study excluded individuals with incomplete records, those with other malignancies, active infections during the assessment period, patients undergoing steroid or immunosuppressive treatment at the time of evaluation, those with autoimmune conditions, and individuals who had received blood transfusions within the month before admission. Based on CECT scans of the abdomen, pelvis, and thorax or PET scans, the study cohort was categorized into two groups: Metastatic and non-metastatic RCC. All patients in the nonmetastatic RCC group underwent laparoscopic/open radical or partial nephrectomy. The metastatic RCC group underwent cytoreductive nephrectomy, immunotherapy, and palliative therapy in some cases.
Patient demographics and clinical, pathological, and laboratory data were collected. Neutrophil-lymphocyte ratio, PLR, SIRI, and SII were calculated from the laboratory data and compared between the two groups. The NLR was determined by dividing the absolute neutrophil count by the absolute lymphocyte count. In contrast, the PLR was calculated by dividing the platelet count by the absolute lymphocyte count. The SIRI was derived by multiplying the absolute neutrophil and monocyte counts and then dividing the result by the absolute lymphocyte count. To obtain the SII, the platelet count was multiplied by the absolute neutrophil count, and this product was then divided by the absolute lymphocyte count.
Statistical analysis: The sample size required for the statistical significance of the study was estimated using the Statulator online software (16). Sample For normally distributed data, continuous variables were presented as mean and standard deviation, while skewed distributions used median and interquartile ranges. Categorical variables, however, were expressed as frequencies and percentages. Normally distributed continuous variables were analyzed using Student's t-test, whereas the Mann-Whitney U test was employed for those with skewed distributions. The chi-square test was applied to evaluate the significance of various factors in predicting metastasis presence or absence in RCC. Renal cell carcinoma curves were employed to assess the capability of NLR, PLR, SIRI, and SII in predicting metastasis presence or absence in RCC, with calculations made for area under the curve (AUC), sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV). The Youden Index was utilized to determine cut-off values for parameter categorization. The relationship between significant factors was examined using binomial logistic regression, incorporating univariate and multivariate analysis. Odds ratios were calculated for variables with a 95% confidence interval. Statistical analyses were conducted using Jamovi 2.5 software, with statistical significance defined as a P-value below 0.05 (17).
4. Results
Of the total 124 patients with RCC, the study included 91 RCC patients, comprising 61 males and 30 females, with an average age of 56.2 ± 11.2 years, after meeting exclusion and inclusion criteria. Table 1 displays the clinicopathological features of the cohort. Among the participants, 25 (27.5%) were identified as having metastatic RCC, whilst 66 (72.5%) had non-metastatic RCC. The classification table has been shown as Table 2. The mean age was comparable between the non-metastatic and metastatic groups (56.2 ± 10.2 vs 56.12 ± 13.75, P = 0.97). The patients’ average Body Mass Index (BMI) was 29.12 + 8.16. In the non-metastatic group, the BMI was 29.86 + 5.2, whereas in the metastatic group, it was 27.12 + 6.14 (P ≥ 0.05).
| Variables | Group 1 [Non-metastatic (n = 66)] | Group 2 [Metastatic (n = 25)] | P-Value |
|---|---|---|---|
| Age | 56.2 ± 10.2 | 56.12 ± 13.75 | 0.97 |
| Male | 41 (62) | 20 (80) | 0.10 |
| Smoking | 22 (33) | 9 (36) | 0.81 |
| Diabetes mellitus | 17 (25) | 7 (28) | 0.82 |
| Hypertension | 40 (60) | 12 (48) | 0.27 |
| Cardiovascular diseases | 8 (12) | 2 (8) | 0.57 |
| Haemoglobin (mg/dL) | 12.89 ± 1.99 | 10.87 ± 2.27 | < 0.001 b |
| White blood cell count (103/µm) | 7500 (5900 - 8800) | 7400 (6500 - 9000) | 0.32 |
| Platelet count (103/µm) | 271 (239 - 330) | 319 (246 - 425) | 0.16 b |
| ANC (103/µm) | 4.93 ± 1.56 | 5.34 ± 2.13 | 0.31 |
| ALC (103/µm ) | 1976 (1699 - 2360) | 1748 (1268 - 2302) | 0.04 b |
| AMC (103/µm) | 576 (491 - 719) | 803 (605 - 937) | 0.002 b |
| NLR | 2.24 (1.82 - 2.99) | 3.12 (2.07 - 4.72) | 0.047 b |
| PLR | 133 (108 - 162) | 184 (143 - 228) | 0.004 b |
| SIRI ($) | 1.46 (1.08 - 1.98) | 2.05 (1.39 - 3.43) | 0.006 b |
| SII ($) | 626 (482 - 918) | 1072 (638 - 1556) | 0.005 b |
Abbreviations: SIRI, Systemic Inflammatory Response Index; SII, Systemic Immune-Inflammatory Index; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; AMC, absolute monocyte count; NLR, neutrophil lymphocyte ratio; PLR, platelet lymphocyte ratio.
a Values are expressed as No. (%) or mean ± SD (median and interquartile range).
b P-value < 0.05.
| Observed | Predicted | % Correct | |
|---|---|---|---|
| Metastatic | Non-metastatic | ||
| Metastatic | 11 | 14 | 44.0 |
| Non-metastatic | 1 | 65 | 98.5 |
a The cut-off value is set to 0.5.
On assessing the comorbidities of the patients, 56 % were found to have hypertension, 26% had diabetes mellitus, and 11% had cardiovascular disease. Patients were found to have unilateral tumors in both groups. In the non-metastatic group, radical nephrectomy was performed in 45 (68.2%) patients, whereas 21 (31.8%) patients underwent partial nephrectomy. Laparoscopic surgery was done in 15 (33.3%) patients out of 45 who underwent radical nephrectomy. The incidence of various RCC subtypes noted in histopathology was clear cell at 81%, Chromophobe at 8%, Papillary at 6 %, and other subtypes at 5%. Three patients in the metastatic RCC group underwent cytoreductive nephrectomy, and four patients had core needle biopsy. No pathological confirmation was available for the remaining patients in the metastatic RCC group, and diagnosis was based on imaging.
In the metastatic group, haemoglobin and absolute lymphocyte count were significantly lower, and absolute monocyte count was significantly higher than in the non-metastatic group. No statistical significance was observed when comparing total leucocyte count and absolute neutrophil count.
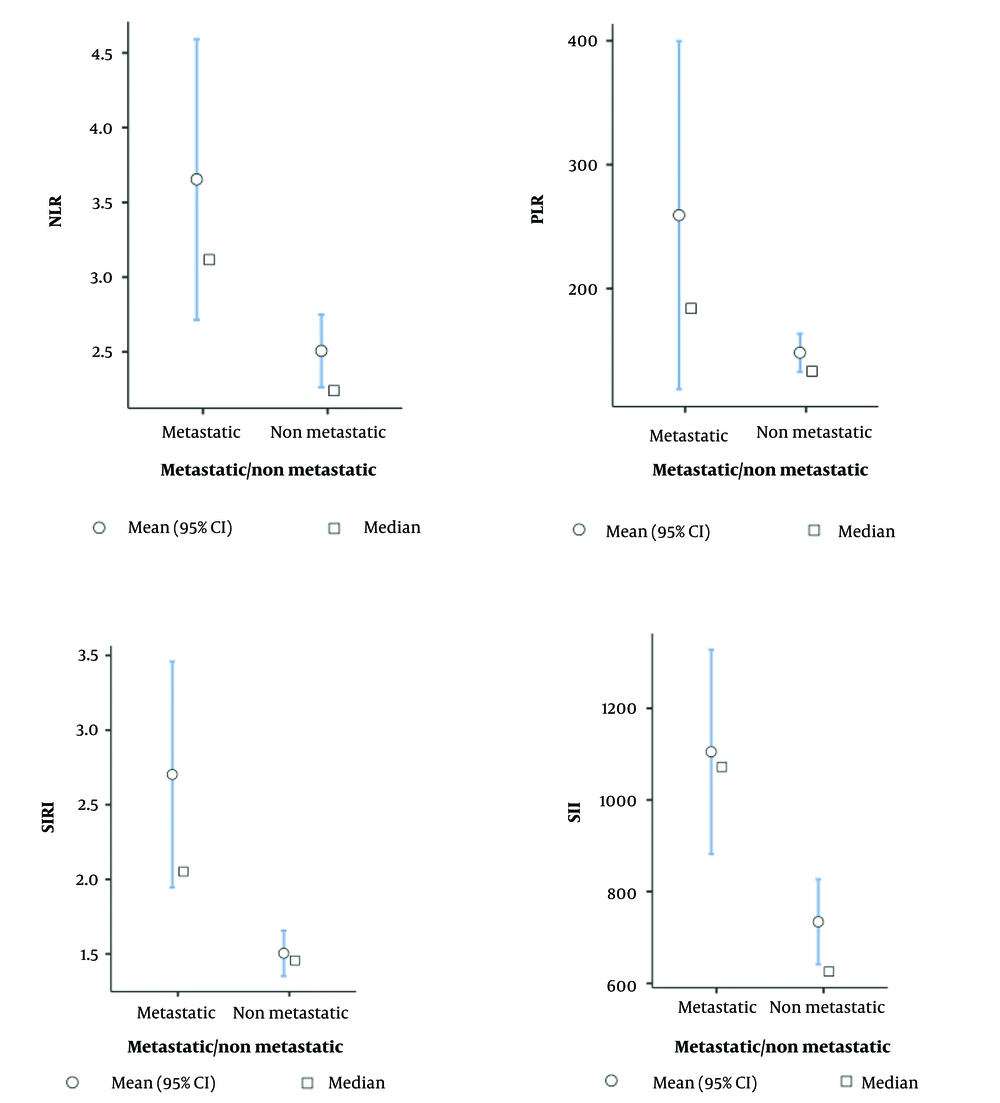
For the non-metastatic and metastatic groups, the SIRI values showed median values of 1.46 and 2.05, respectively, with corresponding mean ± standard deviations of 1.5 ± 0.63 and 2.7 ± 1.93 (P = 0.006). The SII values exhibited medians of 626 and 1072 for the non-metastatic and metastatic groups, respectively, with mean ± standard deviation of 734 ± 384 and 1105 ± 568.5 (P = 0.005). Regarding NLR values, the non-metastatic and metastatic groups displayed medians of 2.24 and 3.12, respectively, with mean ± standard deviation of 2.51 ± 1.003 and 3.65 ± 2.39 (P = 0.047). The PLR values demonstrated medians of 133 and 184 for the non-metastatic and metastatic groups, respectively, with mean ± standard deviation of 148 ± 63 and 259 ± 358 (P = 0.006), as depicted in Figure 1.
Descriptive plots for neutrophil lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), Systemic Inflammatory Response Index (SIRI) and Systemic Immune-Inflammatory Index (SII) with statistical analysis done using the Student’s t-test. P < 0.05 was considered as statistically significant.
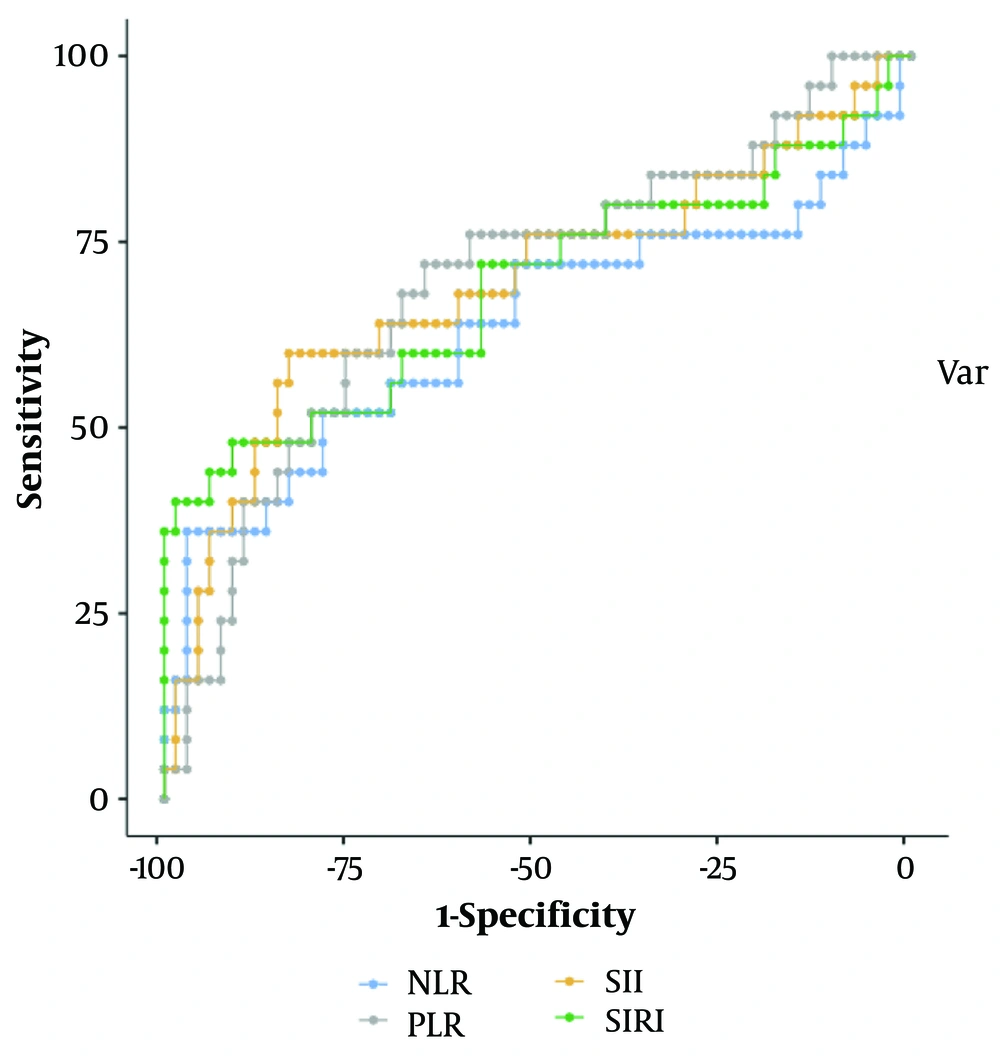
In Figure 2, the performance of NLR, PLR, SIRI, and SII in predicting the presence of metastasis in RCC is assessed using an ROC curve analysis. Table 3 indicates each marker's optimal cut-off value, sensitivity, specificity, PPV, and NPV. The area under the curve for SIRI and SII was 0.687 and 0.693, respectively.
Abbreviations: SIRI, Systemic Inflammatory Response Index; SII, Systemic Immune, Inflammatory Index;; NLR, neutrophil lymphocyte ratio; PLR, platelet lymphocyte ratio; AUC, under the curve; PPV, positive predictive value; NPV, negative predictive value.
a Values are expressed as % unless otherwise indicated.
b P-value < 0.05.
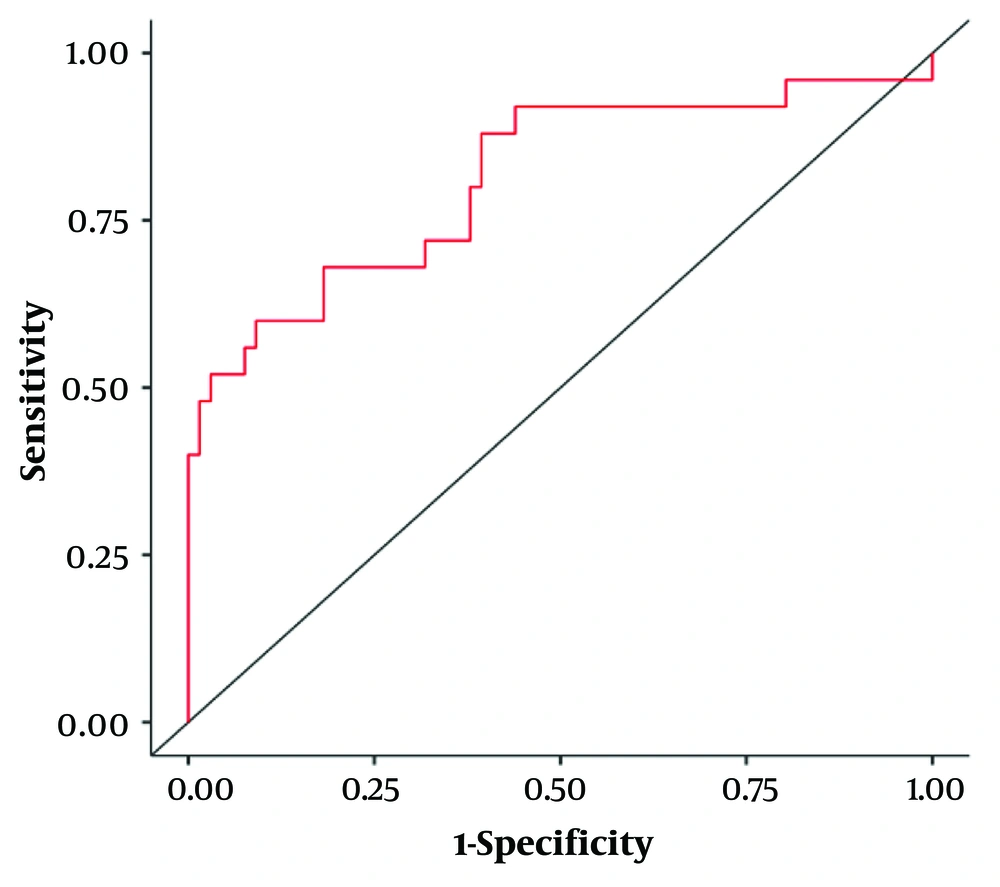
A binary logistic regression was performed to evaluate whether NLR, PLR, SIRI, and SII could be used individually to predict metastasis in RCC and the accuracy of their combined predictions, as shown in Table 4. The overall model was significant, with a 𝛘2 (4) value of 29.5 and a P-value of < 0.001 (Table 5). The combined receiver operating characteristic curve of NLR, PLR, SIRI, and SII is displayed in Figure 3. Across both groups of outcomes, 83.5% of cases were correctly classified.
Abbreviations: NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; SIRI, Systemic Inflammatory Response Index; SII, Systemic Immune-Inflammatory Index.
a P-value < 0.05.
| Model | Deviance | AIC | BIC | R²McF | R²CS | R²N | Overall Model Test | ||
|---|---|---|---|---|---|---|---|---|---|
| χ² | df | P | |||||||
| 1 | 77.5 | 87.5 | 100 | 0.276 | 0.277 | 0.401 | 29.5 | 4 | < 0.001 |
a P-value less than 0.5 was considered as significant.
5. Discussion
In RCC, about 30% of patients present with metastatic disease; hence, it is crucial to identify factors with good predictive accuracy in the early detection of metastases (18). In RCC, the immune system is highly activated. Hence, the inflammatory markers can be good predictors for future metastasis detection and prognostication. Inflammatory mediators and responses have been associated with RCC, and an increase in neutrophils, platelets, and tumor-associated macrophages is associated with poor prognosis and advanced disease (9, 10). In this study, NLR, PLR, SIRI, and SII derived from complete blood counts at admission were evaluated for their predictive efficacy of metastasis in RCC and were found effective.
In our study, NLR was significantly elevated in patients with metastatic RCC compared to patients without metastasis. An increase in NLR is associated with an increased risk of future recurrence or disease progression in non-metastatic disease, as shown in a meta-analysis by Hu et al., as well as shortened overall survival in patients with advanced metastatic RCC as shown by Simonaggio et al. (19, 20). Research has shown that the tumour microenvironment and systemic inflammatory response play crucial roles in the development and progression of tumours. Additionally, neutrophils could indirectly alter the tumour microenvironment, thereby facilitating cancer metastasis (21-23). Contrarily, lymphocytes that reflect cell-mediated immunity are associated with anti-tumor immune responses, and their low counts are associated with tumor progression, which supports our association of high NLR values with metastasis (24).
Similar to NLR, PLR is also an inflammatory haematological ratio studied in detail. In our current study, PLR was significantly elevated in metastatic RCC compared to non-metastatic RCC. Studies have shown that platelets are important in the progression and dissemination of malignancies (25). Platelets release various growth factors that contribute to the progression and spread of tumours. These include vascular endothelium tumour growth, platelet-activating factor, and platelet-derived growth factor, which collectively promote tumour development and metastasis (26). Yuk et al. showed the association of high values of PLR with poor survival in metastatic RCC (27). Ouanes et al., in their cross-sectional study, showed a significant correlation between elevated PLR and poor prognosis of aggressive nonmetastatic RCC. This correlation was also associated with aggressive disease and metastatic disease (28).
The significant decrease in hemoglobin among the metastatic RCC patients can be attributed to the increased incidence of persistent gross hematuria in that group, and it is a well-known criterion in the international metastatic renal cell carcinoma database consortium (IMDC) (29).
A recent study by Ari et al. assessed the effectiveness of SIRI and SII in predicting metastasis in RCC, concluding that both parameters are valuable predictors (15). Their research revealed median SIRI values of 1.26 and 2.1 for non-metastatic and metastatic groups, respectively (P < 0.05). Our findings align with these results, showing median SIRI values of 1.46 and 2.05, with mean ± standard deviation of 1.5 ± 0.63 and 2.7 ± 1.93, respectively (P = 0.006). Regarding SII, their study reported median values of 566 and 1434 for non-metastatic and metastatic RCC, with corresponding mean ± standard deviation of 870 ± 1019 and 1537 ± 917, respectively (P < 0.001). In comparison, our research yielded median SII values of 626 and 1072 for non-metastatic and metastatic groups, with mean ± standard deviation of 734 ± 384 and 1105 ± 568.5, respectively (P = 0.005). The difference in median values can be attributed to factors like tumor characteristics and host immune response. However, they have not compared other parameters like NLR and PLR.
In our study, we evaluated the role of NLR, PLR, SIRI, and SII and found all of them significant predictors of metastasis. The binary logistic regression model showed that all four parameters efficiently predict metastasis, and the combined receiver operating characteristic curve of NLR, PLR, SIRI, and SII shows better predictive ability compared to the individual parameters. As far as we know, no other study has incorporated all these four parameters together to predict metastasis in RCC.
5.1. Limitations
Study findings can not be generalized due to the study's single-center design and small sample size. Hematologic indices are easy to measure, but various factors can affect their effectiveness. Also, patient follow-up data were not taken to assess overall survival. A multicentric, prospective study with follow-ups in the future could address this limitation.
5.2. Conclusions
The NLR, PLR, SIRI, and SII are reliable in predicting metastasis in RCC and, when combined together, enhance predictive accuracy. These reliable predictors of metastasis may help improve patient outcomes by facilitating early detection, enhancing prognostication, and managing RCC. Further research is required to validate and integrate these tools into routine clinical practice.