1. Background
According to GLOBOCAN’s recent report, cervical cancer (CxCa) ranks 8th in incidence with 661 021 cases and 9th in mortality with 348 189 deaths worldwide (1). The CxCa has no symptoms in the early stages and usually presents in advanced stages with abnormal vaginal bleeding, pelvic pain, or pain during sexual intercourse (2).
The mainstay of CxCa treatment is surgery, radiotherapy, and/or chemotherapy, tailored to the disease and patient-specific factors. Radiotherapy for CxCa is generally performed in two ways: External beam Radiotherapy (EBRT) and brachytherapy (BT). Two common methods of BT for CxCa are intracavitary and interstitial. Brachytherapy provides local irradiation and allows for the adaptation of the prescribed dose to the volume of the treatment target, minimizing high doses to surrounding normal tissues, called organs at risk (OAR). However, this aspect of BT can be a double-edged sword. If high precision is not achieved during treatment, it may result in inadequate irradiation of the target volume or excessive irradiation of the surrounding OAR. As such, image-guided adaptive brachytherapy (IGABT) is commonly used because it allows for personalized and optimized treatment. By using three-dimensional imaging, IGABT helps accurately locate the tumor and OAR to improve the treatment results (3).
International standards recommend a cumulative dose of 85 - 90 Gy to the high-risk clinical target volume (CTVHR) for CxCa treatment (4). Due to differing dose rates in EBRT and BT, the EQD2 (Equivalent Dose in 2 Gy fractions) formula can be used to combine these doses accurately. However, this method faces several limitations in terms of tissue and spatial heterogeneities in dose distribution (5, 6). Currently, radiotherapy centers refer patients to BT with details of the external dose and treatment sessions, but this method lacks precision in calculating the cumulative dose.
2. Objectives
This study aims at leveraging advanced BT software to calculate dose-volume histogram (DVH) parameters and compare them with traditional manual methods.
3. Methods
3.1. Brachytherapy Procedure and Reverse Treatment Planning
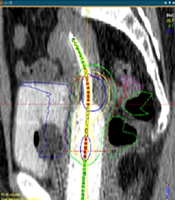
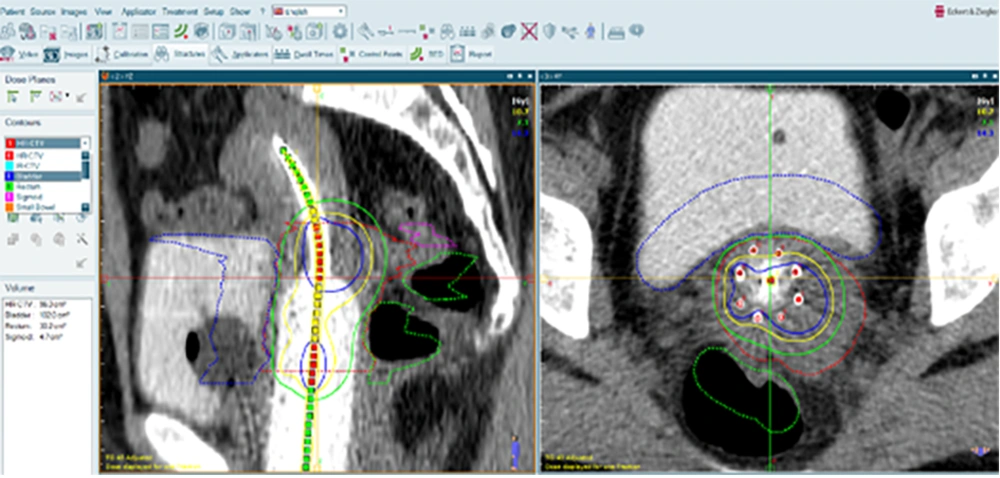
The study protocol was approved by the IRB of [blinded for review]. This cross-sectional study was conducted at [blinded for review] in June 2024. Patients were recruited between August 2023 and May 2024. Patients with histopathological diagnosis of CxCa, who have undergone EBRT and BT, were considered eligible. The BT procedure began with the physician diagnosing the need for interstitial BT and identifying the target area on the patient’s computed tomography (CT) or magnetic resonance imaging (MRI) images. The patient was, then, placed under general or spinal anesthesia in the operating room, where the implant pattern was attached to the perineum. Guided by the CT images and in consultation with the BT physicist, the physician inserted hollow plastic catheters through the implant pattern. The patient was, then, moved to the recovery room and subsequently to the imaging room for CT evaluation of the catheter positions. The physician reviewed the CT images to confirm the correct placement of the catheters and adjusted or added new ones if necessary. Finally, the patient was transferred to the radiation room, where radioactive sources were loaded into the catheters for treatment. After the treatment, the catheters were safely removed. Before starting the irradiation, the patient’s CT images in DICOM format were entered into the SagiPlan® software to design the reverse treatment plan. The target tissue and surrounding healthy tissues were contoured, and the location of the applicator was determined (Figure 1). Based on the desired radiation range specified by the attending physician, the reverse treatment plan was designed to ensure the highest dose to the target tissue and the lowest dose to the healthy tissues. Adjustments were made as necessary to optimize the treatment, and the system was, then, prepared for irradiation.
Identification of the target tissue, organs at risk (OAR), and the desired applicator for treatment. The dotted green, blue, and pink areas represent the rectum, bladders, and sigmoid, respectively. The dotted red area indicates the target tissue. The continuous green, yellow, and blue lines illustrate the dose distribution.
3.2. Brachytherapy Dose Calculation
In SagiPlan® software version 2.2, the AAPM TG-43 formula was used to calculate the dose distribution. This formula assumes a complete water environment and does not consider tissue heterogeneity. The formula is defined as follows.
Where:
D(r): Represents the absorbed dose at a distance r from the radioactive source.
Sk: Is the dose conversion factor depending on the radioactive source and the environment.
φ(r): Represents the dose rate distribution at distance r.
A(r): Is a geometric-radiological factor that accounts for the characteristics of the source and the environment.
r: The distance from the radioactive source.
To load the EBRT plan, RT Struct, RT Dose, and RT Plan data were entered into the software. The reverse treatment design capability was used to adjust the treatment plan for each session based on the maximum dose for each tissue. The BT plan varied for each session, and doses were recorded for 3 sessions. Using the BED/EQD2 Summation feature in SagiPlan® software, the cumulative dose from both EBRT and BT was determined.
3.3. Cumulative Dose Calculation
To combine the doses from EBRT and BT, considering their radiobiological differences, the EQD2 formula should be used. This formula converts the BT dose to an equivalent dose of 2 Gy per fraction (EQD2). Doing so allows for the algebraic addition of the EBRT dose to determine the cumulative dose received by healthy tissues and the target volume. The following formula is applied in the manual method.
Where: BED (biologically effective dose) is calculated, using the following formula:
α/β: The sensitivity of tissues to fractionated radiation doses.
d: Per fraction radiation dose.
n: Fraction numbers.
The EQD2 method is utilized under the assumption of a ‘worst-case scenario’. This implies that it is presumed that identical regions of tissue—for instance, the rectum—receive maximal dosage during both EBRT and successive BT sessions. Such an approach does not consider potential variations in tissue positioning or morphology between these treatment sessions. Notably, during BT procedures, the insertion of applicators into a patient’s body significantly influences tissue wall positioning and condition.
In our study, we utilized SagiPlan’s advanced feature for automatic dose summation of EBRT and BT. This capability, known as BED/EQD2 Calculation, allows for an integrated assessment of cumulative DVH parameters. The process begins within the BED/EQD2 Calculation window, where additional studies pertinent to the patient’s treatment can be selected through the Additional BED Studies window. These are, then, loaded into SagiPlan® and matched with corresponding structures via drag-and-drop functionality in the BED Structure Matching window. It is crucial to note that only studies belonging to the same patient and being either sheer high-dose-rate (HDR) or DICOM Import Studies are eligible for this summation. Furthermore, imported DICOM studies must have clear dose per fraction and number of fractions data. However, caution must be exercised when interpreting results; due to potential variations in volume overlap across HDR treatments and additional BED studies, calculated values represent maximum scenarios that may not reflect actual clinical situations. More information is provided in the SagiPlan Manual Guide version 2.2 (7).
3.4. Ethical Considerations
The study was a retrospective analysis of medical records, presented anonymously. As it did not involve direct patient interaction or intervention, formal ethical approval was not required. However, we ensured that all data were handled following applicable privacy regulations and institutional guidelines to maintain confidentiality and uphold ethical standards.
3.5. Statistical Analysis
Descriptive statistics, including minimum, maximum, and standard deviation (SD) dose values were computed for target tissue and OARs. To determine the significance of the differences between the two methods, paired t tests were performed for normally distributed data. The paired t-test was selected because it is appropriate for comparing two related samples, such as the manual and automatic calculations for the same patients in this study. For data that did not meet the normality assumption, the Wilcoxon signed-rank test was used as a non-parametric alternative. P-values were calculated to assess the statistical significance of the differences between the manual and automatic methods. The significance level was set to 0.05. The IBM SPSS Statistics® (ver. 26) was applied for statistical analysis.
4. Results
In this section, we present and evaluate the dosimetric results for tissues at risk (rectum, bladder, and sigmoid) and the target tissue in 32 CxCa samples treated with EBRT and BT. Tables 1 and 2 illustrate the comparison of doses received by OARs and the target tissue in EBRT, calculated both manually (EQD2) and automatically (SagiPlan®).
| Patient No. | EBRT Fractions | Dose per Fraction (Gy) | D90 (Gy) (SagiPlan®) | D90 (Gy) (EQD2) | Percentage of Relative Error |
|---|---|---|---|---|---|
| 1 | 25 | 1.8 | 44.37 | 44.3 | 0.15 |
| 2 | 28 | 1.8 | 48.91 | 49.6 | -1.41 |
| 3 | 25 | 1.8 | 45.40 | 44.3 | 2.42 |
| 4 | 25 | 1.8 | 43.45 | 44.3 | -1.95 |
| 5 | 25 | 1.8 | 44.79 | 44.3 | 1.09 |
| 6 | 26 | 1.8 | 46.01 | 46.0 | 0.02 |
| 7 | 25 | 1.8 | 43.78 | 44.3 | -1.18 |
| 8 | 25 | 2.0 | 48.95 | 50.0 | -2.14 |
| 9 | 25 | 1.8 | 44.69 | 44.3 | 0.87 |
| 10 | 28 | 1.8 | 49.88 | 49.6 | 0.56 |
| 11 | 28 | 1.8 | 49.18 | 49.6 | -0.85 |
| 12 | 25 | 1.8 | 43.79 | 44.3 | -1.16 |
| 13 | 25 | 1.8 | 43.46 | 44.3 | -1.93 |
| 14 | 25 | 1.8 | 44.80 | 44.3 | 1.11 |
| 15 | 25 | 1.8 | 45.54 | 44.3 | 2.72 |
| 16 | 25 | 1.8 | 46.33 | 44.3 | 4.38 |
| 17 | 25 | 1.8 | 45.17 | 44.3 | 1.92 |
| 18 | 25 | 1.8 | 44.32 | 44.3 | 0.04 |
| 19 | 27 | 1.8 | 46.62 | 47.8 | -2.53 |
| 20 | 25 | 1.8 | 44.49 | 44.3 | 0.42 |
| 21 | 25 | 1.8 | 44.81 | 44.3 | 1.13 |
| 22 | 25 | 1.8 | 43.79 | 44.3 | -1.16 |
| 23 | 28 | 1.8 | 49.06 | 49.6 | -1.10 |
| 24 | 20 | 2.5 | 52.45 | 52.1 | 0.66 |
| 25 | 25 | 1.8 | 49.24 | 44.3 | 10.03 |
| 26 | 28 | 1.8 | 49.54 | 49.6 | -0.12 |
| 27 | 27 | 1.8 | 48.05 | 47.8 | 0.52 |
| 28 | 25 | 1.8 | 43.93 | 44.3 | -0.84 |
| 29 | 25 | 1.8 | 43.88 | 44.3 | -0.95 |
| 30 | 25 | 1.8 | 46.33 | 44.3 | 4.38 |
| 31 | 25 | 1.8 | 44.49 | 44.3 | 0.42 |
| 32 | 25 | 1.8 | 44.80 | 44.3 | 1.11 |
Abbreviation: EBRT, external beam radiotherapy.
| Patient No. | EBRT Fractions | Dose per Fraction (Gy) | D2 cc (Gy) (EQD2) | Rectum (D2 cc) | Bladder (D2 cc) | Sigmoid (D2 cc) | |||
|---|---|---|---|---|---|---|---|---|---|
| D2cc (Gy) (SagiPlan®) | Percentage of Relative Error | D2cc (Gy) (SagiPlan®) | Percentage of Relative Error | D2cc (Gy) (SagiPlan®) | Percentage of Relative Error | ||||
| 1 | 25 | 1.8 | 43.2 | 45.54 | 5.13 | 46.40 | 6.89 | 45.46 | 4.97 |
| 2 | 28 | 1.8 | 48.4 | 50.8 | 4.72 | 51.49 | 6.00 | 50.72 | 4.57 |
| 3 | 25 | 1.8 | 43.2 | 47.26 | 8.59 | 46.13 | 6.35 | 45.78 | 5.63 |
| 4 | 25 | 1.8 | 43.2 | 44.18 | 2.21 | 46.43 | 6.95 | 42.82 | -0.88 |
| 5 | 25 | 1.8 | 43.2 | 45.30 | 4.63 | 45.91 | 5.90 | 45.67 | 5.40 |
| 6 | 26 | 1.8 | 44.9 | 47.76 | 5.98 | 47.90 | 6.26 | 57.54 | 21.96 |
| 7 | 25 | 1.8 | 43.2 | 44.04 | 1.90 | 47.44 | 8.93 | 54.66 | 20.96 |
| 8 | 25 | 2.0 | 50.0 | 52.16 | 4.14 | 51.58 | 3.06 | 50.18 | 0.35 |
| 9 | 25 | 1.8 | 43.2 | 46.10 | 6.29 | 53.50 | 19.25 | 58.66 | 26.35 |
| 10 | 28 | 1.8 | 48.4 | 50.30 | 3.77 | 51.66 | 6.31 | 52.37 | 7.58 |
| 11 | 28 | 1.8 | 48.4 | 50.33 | 3.83 | 50.74 | 4.61 | 49.57 | 2.36 |
| 12 | 25 | 1.8 | 43.2 | 44.20 | 2.26 | 45.03 | 4.06 | 45.33 | 4.69 |
| 13 | 25 | 1.8 | 43.2 | 44.24 | 2.35 | 44.9 | 3.78 | 43.55 | 0.80 |
| 14 | 25 | 1.8 | 43.2 | 46.66 | 7.41 | 47.29 | 8.64 | 48.08 | 10.14 |
| 15 | 25 | 1.8 | 43.2 | 45.86 | 5.80 | 46.02 | 6.12 | 46.02 | 6.12 |
| 16 | 25 | 1.8 | 43.2 | 47.31 | 8.68 | 47.38 | 8.82 | 47.98 | 9.96 |
| 17 | 25 | 1.8 | 43.2 | 45.02 | 4.04 | 47.01 | 8.10 | 48.04 | 10.07 |
| 18 | 25 | 1.8 | 43.2 | 44.88 | 3.74 | 43.91 | 1.61 | 45.30 | 4.63 |
| 19 | 27 | 1.8 | 46.7 | 47.33 | 1.33 | 48.95 | 4.59 | 48.47 | 3.65 |
| 20 | 25 | 1.8 | 43.2 | 45.59 | 5.24 | 46.41 | 6.91 | 46.71 | 7.51 |
| 21 | 25 | 1.8 | 43.2 | 44.06 | 1.95 | 44.68 | 3.31 | 45.13 | 4.27 |
| 22 | 25 | 1.8 | 43.2 | 44.61 | 3.16 | 44.88 | 3.74 | 44.31 | 2.50 |
| 23 | 28 | 1.8 | 48.4 | 47.04 | -2.89 | 51.71 | 6.40 | 49.35 | 1.92 |
| 24 | 20 | 2.5 | 55.0 | 59.66 | 7.81 | 60.06 | 8.42 | 59.99 | 8.31 |
| 25 | 25 | 1.8 | 43.2 | 51.86 | 16.69 | 53.87 | 19.80 | 51.68 | 16.40 |
| 26 | 28 | 1.8 | 48.4 | 50.18 | 3.54 | 50.83 | 4.78 | 48.97 | 1.16 |
| 27 | 27 | 1.8 | 46.7 | 49.71 | 6.05 | 50.08 | 6.74 | 61.95 | 24.61 |
| 28 | 25 | 1.8 | 43.2 | 44.96 | 3.91 | 45.39 | 4.82 | 44.72 | 3.39 |
| 29 | 25 | 1.8 | 43.2 | 44.05 | 1.92 | 44.97 | 3.93 | 44.76 | 3.48 |
| 30 | 25 | 1.8 | 43.2 | 47.31 | 8.68 | 47.38 | 8.82 | 47.98 | 9.96 |
| 31 | 25 | 1.8 | 43.2 | 45.59 | 5.24 | 46.41 | 6.91 | 46.71 | 7.51 |
| 32 | 25 | 1.8 | 43.2 | 46.66 | 7.41 | 47.29 | 8.64 | 48.08 | 10.14 |
Abbreviation: EBRT, external beam radiotherapy.
As expected, according to the EQD2 formula, the EBRT dose values are consistent in different OARs. In other words, the received doses for the rectum, bladder, and sigmoid are the same. However, using the SagiPlan® treatment design software with the BED/EQD2 Summation function different values are observed for different OARs (Table 2).
In the automatic method, we first entered the patient’s EBRT plan into the software. Using the inverse planning function, we then created the plan for the BT sessions. Finally, using the BED/EQD2 summation function, the calculations were completed, and the dose values for all desired tissues were displayed. These values are also effective in calculating the total dose received by these tissues. Tables 3 and 4 compare the doses received by the OARs and the target tissue in both external and internal radiation therapy (i.e., total dose).
| Patient No. | EBRT Fractions | Dose per Fraction (Gy) | D90 (Gy) (SagiPlan®) | D90 (Gy) (EQD2) | Percentage of Relative Error |
|---|---|---|---|---|---|
| 1 | 25 | 1.8 | 72.40 | 72.2 | 0.27 |
| 2 | 28 | 1.8 | 82.29 | 83.1 | -0.98 |
| 3 | 25 | 1.8 | 92.43 | 91.4 | 1.11 |
| 4 | 25 | 1.8 | 79.44 | 80.2 | -0.95 |
| 5 | 25 | 1.8 | 90.65 | 89.9 | 0.82 |
| 6 | 26 | 1.8 | 77.86 | 77.7 | 0.20 |
| 7 | 25 | 1.8 | 79.06 | 79.4 | -0.43 |
| 8 | 25 | 2.0 | 77.69 | 78.8 | -1.42 |
| 9 | 25 | 1.8 | 101.68 | 101.3 | 0.37 |
| 10 | 28 | 1.8 | 89.97 | 89.7 | 0.30 |
| 11 | 28 | 1.8 | 86.28 | 86.7 | -0.48 |
| 12 | 25 | 1.8 | 87.51 | 87.8 | -0.33 |
| 13 | 25 | 1.8 | 83.61 | 84.3 | -0.82 |
| 14 | 25 | 1.8 | 85.52 | 84.9 | 0.72 |
| 15 | 25 | 1.8 | 92.40 | 91.2 | 1.29 |
| 16 | 25 | 1.8 | 89.99 | 88.0 | 2.21 |
| 17 | 25 | 1.8 | 90.82 | 89.9 | 1.01 |
| 18 | 25 | 1.8 | 87.61 | 87.7 | -0.10 |
| 19 | 27 | 1.8 | 85.66 | 86.9 | -1.44 |
| 20 | 25 | 1.8 | 78.36 | 78.1 | 0.33 |
| 21 | 25 | 1.8 | 90.28 | 89.7 | 0.64 |
| 22 | 25 | 1.8 | 78.45 | 79.0 | -0.70 |
| 23 | 28 | 1.8 | 89.47 | 89.8 | -0.36 |
| 24 | 20 | 2.5 | 72.11 | 71.8 | 0.42 |
| 25 | 25 | 1.8 | 86.29 | 87.1 | -0.93 |
| 26 | 28 | 1.8 | 89.33 | 89.2 | 0.14 |
| 27 | 27 | 1.8 | 88.00 | 87.7 | 0.34 |
| 28 | 25 | 1.8 | 87.82 | 88.2 | -0.43 |
| 29 | 25 | 1.8 | 84.71 | 85.2 | -0.57 |
| 30 | 25 | 1.8 | 89.99 | 88.0 | 2.21 |
| 31 | 25 | 1.8 | 78.36 | 78.1 | 0.33 |
| 32 | 25 | 1.8 | 85.52 | 84.9 | 0.72 |
Abbreviation: EBRT, external beam radiotherapy.
| Patient No. | EBRT Fractions | Dose per Fraction (Gy) | Rectum (D2 cc) | Bladder (D2 cc) | Sigmoid (D2 cc) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D2cc (Gy) (SagiPlan®) | D2cc (Gy) (EQD2) | Percentage of Relative Error | D2cc (Gy) (SagiPlan®) | D2cc (Gy) (EQD2) | Percentage of Relative Error | D2cc (Gy) (SagiPlan®) | D2cc (Gy) (EQD2) | Percentage of Relative Error | |||
| 1 | 25 | 1.8 | 76.42 | 74.2 | 2.90 | 83.15 | 80.1 | 3.66 | 72.53 | 70.2 | 3.21 |
| 2 | 28 | 1.8 | 77.92 | 75.5 | 3.10 | 92.95 | 88.9 | 4.35 | 77.49 | 75.1 | 3.08 |
| 3 | 25 | 1.8 | 67.48 | 63.4 | 6.04 | 79.99 | 77.2 | 3.48 | 68.89 | 66.3 | 3.75 |
| 4 | 25 | 1.8 | 74.83 | 73.9 | 1.24 | 87.59 | 84.3 | 3.75 | 69.72 | 70.0 | -0.40 |
| 5 | 25 | 1.8 | 74.16 | 72.3 | 2.50 | 79.81 | 77.2 | 3.27 | 76.82 | 74.3 | 3.28 |
| 6 | 26 | 1.8 | 76.02 | 73.1 | 3.84 | 84.94 | 81.7 | 3.81 | 66.94 | 54.3 | 18.88 |
| 7 | 25 | 1.8 | 72.15 | 71.1 | 1.45 | 89.51 | 85.4 | 4.59 | 84.68 | 73.4 | 13.32 |
| 8 | 25 | 2.0 | 72.59 | 70.3 | 3.15 | 75.46 | 73.9 | 2.06 | 66.39 | 66.2 | 0.28 |
| 9 | 25 | 1.8 | 66.32 | 63.3 | 4.55 | 93.72 | 83.2 | 11.22 | 77.33 | 61.9 | 19.95 |
| 10 | 28 | 1.8 | 52.44 | 50.5 | 3.69 | 71.85 | 68.5 | 4.66 | 58.05 | 54.1 | 6.80 |
| 11 | 28 | 1.8 | 72.56 | 70.7 | 2.56 | 85.80 | 83.5 | 2.68 | 54.07 | 52.8 | 2.34 |
| 12 | 25 | 1.8 | 75.24 | 74.3 | 1.24 | 91.95 | 90.3 | 1.79 | 61.37 | 59.4 | 3.21 |
| 13 | 25 | 1.8 | 75.74 | 75.0 | 0.97 | 87.26 | 85.2 | 2.36 | 68.45 | 68.2 | 0.36 |
| 14 | 25 | 1.8 | 79.19 | 75.6 | 4.53 | 86.77 | 82.9 | 4.46 | 79.1 | 74.3 | 6.06 |
| 15 | 25 | 1.8 | 75.65 | 73.1 | 3.37 | 92.86 | 89.8 | 3.29 | 78.58 | 75.7 | 3.66 |
| 16 | 25 | 1.8 | 70.19 | 66.3 | 5.54 | 84.20 | 80.0 | 4.98 | 82.17 | 77.3 | 5.92 |
| 17 | 25 | 1.8 | 69.43 | 67.6 | 2.63 | 92.25 | 88.4 | 4.17 | 80.21 | 75.2 | 6.24 |
| 18 | 25 | 1.8 | 64.62 | 62.8 | 2.81 | 86.52 | 85.6 | 1.06 | 72.87 | 70.7 | 2.97 |
| 19 | 27 | 1.8 | 64.65 | 64.0 | 1.00 | 71.72 | 69.2 | 3.51 | 79.75 | 78.2 | 1.94 |
| 20 | 25 | 1.8 | 58.07 | 55.8 | 3.90 | 73.90 | 70.7 | 4.33 | 74.89 | 71.5 | 4.52 |
| 21 | 25 | 1.8 | 67.11 | 66.3 | 1.20 | 86.33 | 84.9 | 1.65 | 65.6 | 63.6 | 3.04 |
| 22 | 25 | 1.8 | 77.78 | 76.3 | 1.90 | 88.14 | 86.5 | 1.86 | 83.86 | 82.8 | 1.26 |
| 23 | 28 | 1.8 | 61.99 | 63.2 | -1.95 | 81.65 | 78.4 | 3.98 | 74.84 | 73.9 | 1.25 |
| 24 | 20 | 2.5 | 75.07 | 70.4 | 6.22 | 82.84 | 77.7 | 6.20 | 75.46 | 70.5 | 6.57 |
| 25 | 25 | 1.8 | 76.69 | 74.9 | 2.33 | 83.01 | 79.2 | 4.58 | 57.07 | 55.4 | 2.92 |
| 26 | 28 | 1.8 | 76.44 | 74.6 | 2.40 | 82.13 | 79.7 | 2.95 | 59.85 | 59.2 | 1.08 |
| 27 | 27 | 1.8 | 78.32 | 75.3 | 3.85 | 84.51 | 81 | 4.15 | 81.37 | 66.2 | 18.64 |
| 28 | 25 | 1.8 | 56.33 | 54.6 | 3.07 | 78.46 | 76.1 | 3.00 | 60.45 | 58.9 | 2.56 |
| 29 | 25 | 1.8 | 75.28 | 74.4 | 1.16 | 87.20 | 85.2 | 2.29 | 75.64 | 74.1 | 2.03 |
| 30 | 25 | 1.8 | 70.19 | 66.3 | 5.54 | 84.20 | 80 | 4.98 | 82.17 | 77.3 | 5.92 |
| 31 | 25 | 1.8 | 58.07 | 55.8 | 3.90 | 73.9 | 70.7 | 4.33 | 74.89 | 71.5 | 4.52 |
| 32 | 25 | 1.8 | 79.19 | 75.6 | 4.53 | 86.77 | 82.9 | 4.46 | 79.1 | 74.3 | 6.06 |
Abbreviation: EBRT, external beam radiotherapy.
Tables 5 and 6 demonstrate the statistical analysis of the comparison between manual and automatic methods in terms of EBRT and total dose, respectively. The cumulative dose values for the manual (EQD2) and automatic (SagiPlan®) calculations were 69.15 vs. 71.92 Gy for the rectum, 81.87 vs. 84.47 Gy for the bladder, and 69.84 vs.73.15 Gy for sigmoid, which were significantly different between two methods (P < 0.01). However, this difference was not detected in the cumulative dose to the target tissue (P = 0.21).
| EBRT | Target (D90) | Rectum (D2cc) | Bladder (D2cc) | Sigmoid (D2cc) |
|---|---|---|---|---|
| Min | -2.53 | -2.89 | 1.61 | -0.88 |
| Max | 10.03 | 16.69 | 19.80 | 26.35 |
| SD | 2.39 | 3.25 | 3.77 | 6.92 |
| P-value | 0.11 | < 0.01 | < 0.01 | < 0.01 |
Abbreviation: EBRT, external beam radiotherapy.
| EBRT + BT | Target (D90) | Rectum (D2cc) | Bladder (D2cc) | Sigmoid (D2cc) |
|---|---|---|---|---|
| Min | -1.44 | -1.95 | 1.06 | -0.40 |
| Max | 2.21 | 6.22 | 11.22 | 19.95 |
| SD | 0.89 | 1.71 | 1.75 | 5.17 |
| P-value | 0.21 | < 0.01 | < 0.01 | < 0.01 |
Abbreviation: EBRT, external beam radiotherapy.
5. Discussion
In clinical practice, summation of the EBRT and BT dose is crucial to evaluate the total BED to target tissues and OARs (8). To this end, scholars have effort to provide methods to ease access to this endpoint (9). Among these strategies, the linear addition of DVH parameters without image registration has been the global standard for composite dose reporting. This method originated from a time when image guidance was not available, and radiation treatments were simpler. However, with technological advancements, both EBRT and BT have evolved to allow for more precise, volume-based treatment planning, and delivery. Given the complexity of modern treatment techniques, simply adding DVH parameters from EBRT and BT may not accurately reflect the combined dose distribution (10). An alternative approach is deformable image registration (DIR), which aligns datasets from EBRT and image-guided brachytherapy (IGBT) (11). This method can provide a more detailed calculation of combined doses but is still in its early stages and requires further refinement for clinical use.
Another method involves biologic dose summation, which combines physical dose maps from EBRT and each IGBT fraction to generate a composite DVH based on biologically effective doses (12). However, this approach depends on accurate tissue-specific radiobiological parameters, which are not yet fully understood. A potential solution could be combining voxel-based DIR with biologically weighted dose maps for an approximation of total dose accumulation, but this method also needs validation (9). In line with this, many efforts have been made to accurately estimate the overall doses from EBRT and BT plans. However, previous studies utilized either a phantom study or a dosimetric planning study on a few patients (13-15).
The current study was conducted to address these limitations. By examining the results from comparing dose delivery to target tissue and surrounding healthy tissues using both the EQD2 formula and the SagiPlan® treatment design software, we found that outcomes align with expectations based on the EQD2 formula and the number of treatment sessions. In the case of the manual method, the dose values of the rectum, bladder, and sigmoid were the same. However, when using SagiPlan® software’s BED/EQD2 Summation feature, different values emerge. These discrepancies also affect calculations of the total dose delivered to these tissues. A comparison between manual EQD2 calculations (manual) and SagiPlan® software (automatic) for doses delivered to healthy surrounding tissues versus target tissue in both external and internal radiation therapy reveals a relative error percentage across all tissues. Significant differences were noted for the target and surrounding healthy tissues. Notably, sigmoid tissue exhibits the largest deviation among all OARs. To further contextualize our findings, we now turn to a review of similar studies in the literature, which provide additional insights and comparisons.
While the traditional manual method using the simple summation of EQD2 has been referenced (16, 17), there is a growing emphasis on using planning system analysis for a more precise and individualized approach. Modern recommendations advocate for the use of 3D-IGBT combined with advanced planning systems. These systems allow for detailed dose distribution analysis and optimization, ensuring better tumor coverage and sparing of OARs. In an experimental study on 31 patients with CxCa, Gelover et al. evaluated different DVH parameter addition methods for combining EBRT and HDR-BT plans. The findings indicate that the currently recommended method significantly underestimates OAR doses compared to a reference technique. A revised method, which uses patient-specific plans, provided more accurate dose assessments. This highlights the importance of adopting the revised method for cumulative dose calculations to ensure better treatment planning and patient safety (18).
In this study, several potential sources of bias were identified. Selection bias may arise from the specific patient population recruited, which may not be representative of the broader patient demographic; performance bias could occur due to variations in the execution of the BT procedure and the skill levels of the physicians involved; detection bias is a concern if there are inconsistencies in the imaging evaluations of catheter positions. It is essential to recognize these limitations, the validity by which they answer the study questions, and their applicability. Future multi-institutional studies with larger sample sizes using advanced imaging techniques such as MRI or positron emission tomography (PET) or DIR algorithms may enhance dose calculations' accuracy and clinical applicability in cervical cancer radiotherapy.
5.1. Conclusions
In this study, we compared manual calculations, using the EQD2 formula with the automated capabilities of the SagiPlan® treatment design software for calculating cumulative DVH parameters in CxCa radiotherapy. Our findings indicate a significant discrepancy in the dose delivered to surrounding healthy tissues between the two methods. Specifically, manual calculations tend to underestimate the dose, while the SagiPlan® software shows higher dose values. This discrepancy is critical, as it suggests that healthy tissues adjacent to the target area may receive doses exceeding their tolerance thresholds, which is a major concern in radiation therapy, where minimizing exposure to healthy tissue is paramount. This finding can be applied to Radiation Oncology centers to improve the radiotherapy outcomes in patients with CxCa.