1. Background
Skin metastases affect 5% - 30% of individuals with breast cancer and have become an increasingly problematic clinical condition (1). Therefore, there is a growing need for effective treatments to control tumor growth and symptoms. Combining local electric pulses with chemotherapy, electrochemotherapy has become a prominent therapeutic approach (2). Electric pulses are applied to the tumor during electrochemotherapy in order to enhance medication uptake by cancer cells (3). Pain management is necessary as the administered electric pulses cause muscle spasms. Given the invasive nature of this procedure, adequate sedation is crucial for patient comfort and safety (4).
Dexmedetomidine and propofol are widely used sedatives in clinical settings, each offering unique pharmacological profiles that make them suitable for different medical procedures. Dexmedetomidine, an alpha-2 adrenergic agonist, is known for its sedative and analgesic properties with minimal respiratory depression (5), while propofol, a GABA agonist, provides a rapid onset and deep sedation with a relatively short recovery time (6). Previous studies have explored the use of these sedatives across various medical procedures, with mixed results concerning their efficacy and safety (7, 8); however, research specifically addressing sedation for electrochemotherapy in patients with cancer remains limited, highlighting the need for further investigation.
2. Objectives
This study aimed to compare two commonly used sedatives, dexmedetomidine and propofol, to determine which provides optimal sedation for patients undergoing electrochemotherapy for breast cancer skin lesions.
3. Methods
3.1. Patients and Design
Patients aged 18 - 60 years, belonging to ASA groups II, I and III with skin metastases from breast cancer who were referred for electrochemotherapy at the dermatology clinic of Loghman Hakim Hospital in Tehran, Iran, between 2023 and 2024 were enrolled in the study. The criteria for inclusion required participants with symptomatic skin metastases (bleeding, exudation, smell or wound), lack of response to systemic treatment for skin lesions, patient's own preference for electrochemotherapy, multiple skin lesions or lesion larger than 1 cm. All participants provided written informed consent. Patients were excluded if they did not consent to participate, were pregnant or breastfeeding, were allergic to any of the drugs used in the study, had hypertension, hereditary heart diseases, ischemic heart disease, impaired liver or kidney function, or were using beta blockers or sedative drugs. The study was approved by the Medial Ethics Council of Cancer Research Center at Shahid Beheshti University of Medical Science with number: IR.SBMU.CRC.REC.1402.034. The study was conducted according to the Declaration of Helsinki Principles.
3.2. Clinical Procedure
Sequentially numbered, opaque-sealed envelopes with computer-generated random allocations in a 1:1 ratio in balanced blocks of 32 were used to accomplish randomization. Our anesthesia technician prepared the study medicines and covered the IV bag and tubing with an opaque covering to minimize selection biases. The participants and those assessing outcomes were blinded to the evaluation. Before initiation of the procedure, dexmedetomidine injection, was applied with a bolus dose of 0.5 to 1 1 μg/kg followed by a continuous infusion of 0.1 to 1 μg/kg per hour. Propofol was administered with a dose of 25 -75 μg/min/kg. All patients received treatment following the European Standard Operative Procedures for Electrochemotherapy (4). The dosage and method of bleomycin administration were tailored according to the size and number of tumors for intratumoral injections and were adjusted based on the patient's body surface area for intravenous infusions. The procedure was carried out on an outpatient basis, with most patients being discharged after a 24-hour observation period. Peripheral oxygen saturation (SpO2), mean arterial pressure (MAP), heart rate (HR), Ramsay Sedation Scale (RSS) score, Neuropathic Pain Scale (NPS) score and Aldrete score were recorded during the procedure by an anesthesiologist. Vital signs were recorded every 15 minutes through the whole process. The RSS was used to examine the depth of sedation at the beginning and during the electrochemotherapy. Neuropathic Pain Scale was used to assess the pain after surgery. All patients were required to rate their overall satisfaction with the management of their pain (0, poor; 1, good; 2, excellent). The surgeons’ satisfaction with effective pain control, optimal surgical conditions, and minimal side effects during the procedure was also examined (0, poor; 1, good; 2, excellent).
3.3. Statistical Analysis
Data were analyzed using the IBM Statistical Package for Social Sciences v.27 (SPSS Inc., Chicago, IL, USA). A normal distribution of the quantitative data was checked using Shapiro-Wilk test. Repeated-measures analysis of variance was used to compare continuous variables. Variance homogeneity assumption was tested with Levene test. Parametric tests (Independent-samples t-test and post hoc Tukey test) were applied to data of normal distribution and non-parametric tests (Mann-Whiney U-test and Kruskal-Wallis test) were applied to data of questionably normal distribution. Bonferroni estimated marginal means analysis was used for multiple comparison tests. The χ2-test was used to compare discrete variables. The results for all items were expressed as mean ± SD, assessed within a 95% reliance and at a level of P < 0.05 significance. While determining sample size, reference values were received from the study by Benedik et al. (4) and found that minimum of 32 patients were needed in each group to detect a significant difference between groups, with 80% power at a type I error of 0.05. Analyses were performed by G-Power 3.1.7 (Kiel University, Kiel, Germany).
4. Results
The mean age of the patients in the propofol and dexmedetomidine was 51.2 ± 9.4 and 52.9 ± 7.8, respectively (P > 0.05). The mean duration of electrochemotherapy and the recovery time was 129.6 ± 19.2 minutes in the dexmedetomidine group and 130.7 ± 14.8 minutes in the propofol group (P = 0.8).
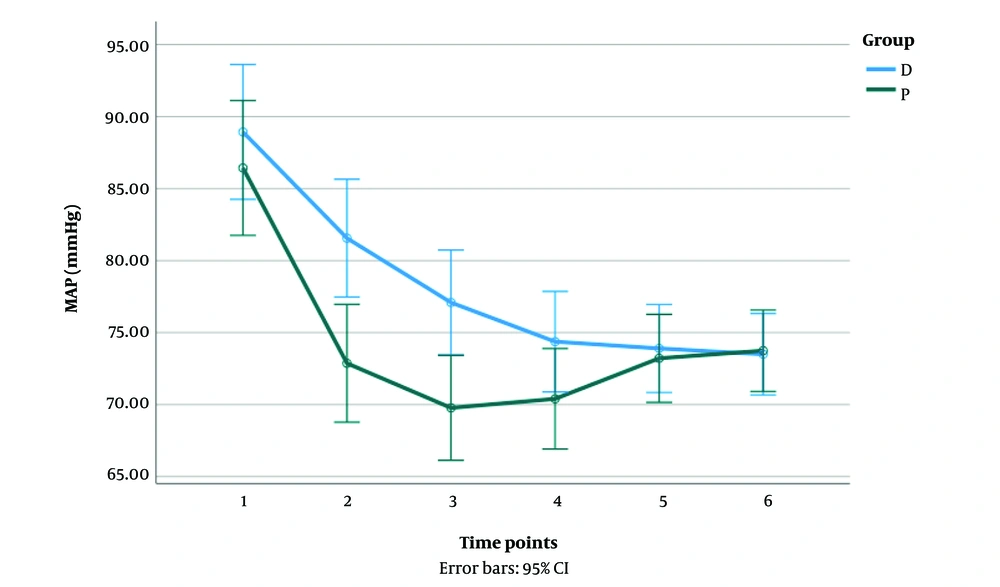
There was a significant interaction between the propofol and the dexmedetomidine groups on MAP reduction [F (2.88, 178.77) = 7.78, P < 0.001]. In the propofol group, patients showed a significant decrease in MAP from time points 1 to 3 (P < 0.05). In the dexmedetomidine group, patients showed a significant reduction in MAP from time points 1 to 6 (P < 0.05). A significant difference in MAP was observed between the groups in the first three time points (P < 0.05) (Figure 1).
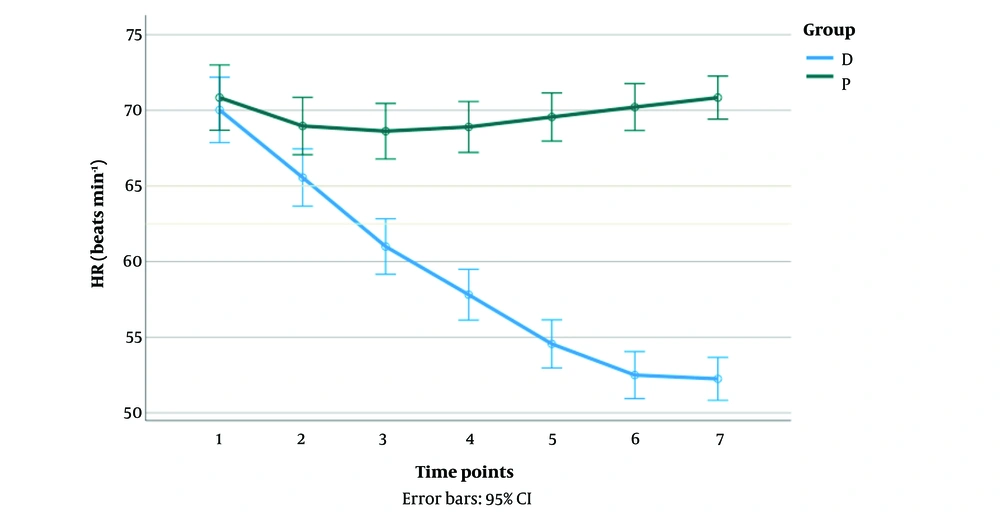
As shown in Figure 2, there was a significant interaction between the propofol and the dexmedetomidine groups on the HR of the patients [F (2.78, 172.7) = 117.54, P < 0.001]. The HR of the patients in the dexmedetomidine group significantly reduced from points 1 to 7 (P < 0.05). In the propofol group, patients showed a significant decrease in HR only in time point 2 (P < 0.05). A significant difference in HR was observed between the groups in time points 2 to 7 (P < 0.05).
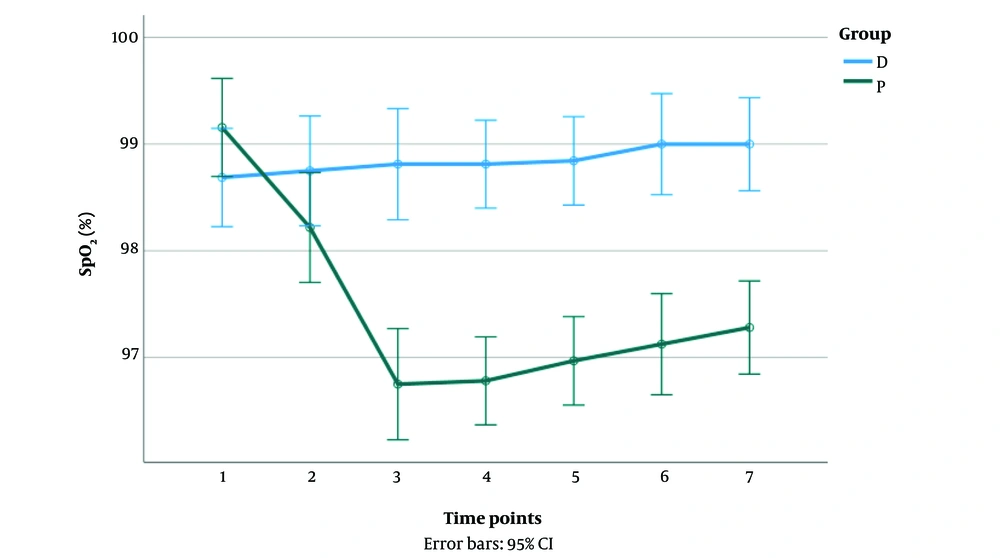
Respiratory depression (SpO2 < 92%) did not occur in any groups of the patients. There was a significant interaction between the groups on SpO2 reduction [F (4.97, 308.55) = 12.94, P < 0.001]. In the dexmedetomidine group, SpO2 reduction was not significant; however, a significant decrease in SpO2 was observed up to time point 3 in the propofol group (P < 0.05). A significant difference in SpO2 was observed between the groups in time points 3 to 7 (P < 0.05) (Figure 3).
All the patients in the dexmedetomidine group had an Alderete score of more than 8; however, only 11 (34.3%) patients in the propofol group had an Alderete score of more than 8, which was significantly different between the groups (P < 0.001).
The NPS score mean was relatively lower in the dexmedetomidine group (0.25 ± 0.5) than in the propofol group (0.38 ± 0.4) but not statistically significant (P = 0.32). The mean RSS score before the initiation of the procedure was lower in the dexmedetomidine group (1.56 ± 0.5) than in the propofol group (1.66 ± 0.4) but not statistically significant (P =0.45). The mean RSS score during the procedure was significantly lower in the dexmedetomidine group (3.53 ± 0.5) than in the propofol group (4.5 ± 0.8) (P < 0.001).
The patients' overall satisfaction was similar between the groups (P > 0.05). The surgeon's satisfaction was better with the dexmedetomidine group than the propofol group (P < 0.001).
5. Discussion
In this study SpO2, MAP, HR, RSS score, NPS score and Aldrete score were evaluated and compared between two groups sedated with dexmedetomidine and propofol. In both groups, MAP decrease was observed; however, a more significant reduction was found in MAP of the propofol group. Arian and Ebert’s study on the efficacy of dexmedetomidine vs propofol in intraoperative sedation, also showed that propofol sedation was associated with lower blood pressures than similarly sedated patients receiving dexmedetomidine throughout surgery (9); Kaygusuz et al. study on the efficacy of dexmedetomidine vs propofol in ESWL, found an equivalent mild reduction in MAPs (10). In our study the HR of the patients in the dexmedetomidine group significantly reduced, while significant reduction in HR was only found in time point 2 in the propofol group, which can be due to vagal mimetic and sympatholytic effects of this drug (5). Oxygen saturation didn’t decrease in the dexmedetomidine group significantly; however, a significant reduction in the SpO2 was observed in the propofol group, which was consistent with Kaygusuz et al. study (10). In our study we found a lower RSS score during the procedure in dexmedetomidine, than propofol and a significant higher Aldrete score in the dexmedetomidine group. Zhang’s study found a faster offset of sedation in the dexmedetomidine group (11); however, Arian and Ebert study, found a slower onset and offset of sedation in dexmedetomidine group (9). The NPS score did not differ significantly between our groups, showing a satisfactory pain control in both groups with overall good satisfaction in both patient groups.
Our study had some limitation. Firstly, administration of the drugs was at different rate and time in each group. Secondly, we didn’t include patients with cardiovascular disease and critically ill patients.
5.1. Conclusions
Both propofol and dexmedetomidine demonstrated satisfactory sedative effect during electrochemotherapy surgery. Therefore, dexmedetomidine may serve as an appropriate alternative to propofol for patients undergoing electrochemotherapy.