1. Background
Colorectal cancer (CRC) is one of the most prevalent cancers affecting the lower gastrointestinal tract. This cancer involves the pathological transformation of normal epithelium to adenomatous polyps and ultimately invasive cancer (1). Colony-stimulating factors (CSF) are glycoproteins that stimulate the proliferation and differentiation of hematopoietic progenitor cells in bone marrow. These factors, produced by various immune and non-immune cells, include macrophage colony-stimulating factor (M-CSF or CSF1), granulocyte M-CSF (GM-CSF or CSF2), and granulocyte colony-stimulating factor (G-CSF or CSF3). They play crucial roles in the survival, proliferation, differentiation, maturation, and functional activation of hematopoietic cells, including monocytes and macrophages (2). Cytokines are a large family of messenger proteins that can induce various cancers. These proteins are mediated by Janus kinases (JAKs). Once activated, these kinases phosphorylate STAT proteins, which regulate genes involved in cell differentiation, proliferation, and apoptosis. Disruption in the JAK/STAT pathway can lead to tumorigenesis and cancer development (3). Activation of the STAT3 signaling pathway promotes human tumor progression by inhibiting apoptosis and inducing cell proliferation, angiogenesis, invasion, and metastasis (4). Additionally, increased activity in the mitogen-activated protein kinase (MAPK) signaling pathway in resistant cell lines leads to increased resistance, and inhibiting this pathway represents a suitable strategy for overcoming cancer cell drug resistance (5). Dysregulation of the MAPK/ERK signaling pathway results in increased cell proliferation, survival, and metastasis in many cancers. For example, high ERK expression induces anti-apoptotic molecules like BCL2, leading to drug resistance in various cancer cells. It has also been reported that signaling pathways such as MAPK/ERK are effective in regulating P-glycoprotein expression. P-glycoprotein, a member of the ABC transporter family, is expressed in normal cells and tissues such as the kidney, liver, colon, and blood-brain barrier for protection against toxic substances (6).
Given the beneficial effects of regular physical activity on health, exercise has been accepted as a safe intervention for improving the quality of life in cancer patients. Recent findings indicate tumor volume reduction following regular exercise (7). Numerous studies have investigated the effects of exercise on preventing severe cancer complications and their outcomes in patients (8). For instance, Zielinski et al. demonstrated that exercise leads to delayed tumor growth (9). Physical activity appears to prevent and treat cancer through multiple mechanisms, including inhibition of cancer cell proliferation, induction of apoptosis, regulation of cancer cell metabolism, and immune system modulation (2). Exercise intensity is one of the most crucial factors determining its role in cancer prevention. The relationship between activity intensity and cancer cell proliferation inhibition has been extensively studied (10). In animal studies, moderate-intensity exercise can prevent cancer cell proliferation and induce apoptosis, indicating the protective effect of moderate-intensity training in cancer prevention. However, while very low-intensity activity has no significant effect on cancer cell proliferation, high-intensity exercise could potentially stimulate cancer cell proliferation (8). Furthermore, increasing antioxidants and reducing free radicals are effective in improving cancer patients.
The increased use of natural antioxidant supplements appears to play a role in reducing oxidative factors. Research has shown that pomegranate's antioxidant power and capacity are stronger than other fruit juices (11). High antioxidant activity has been identified in various parts of pomegranate, including juice, peel, and seeds. This antioxidant activity is largely attributed to its high phenolic compound content (12). Pomegranate fruit can affect various stages of cancer through different pathways, including inhibition of aromatases and cyclooxygenase enzymes, effects on heat shock proteins, leucine zipper transcription factor, and specific protein transcription factors and cell cycle (12). Based on previous and clinical studies conducted by various laboratories worldwide, pomegranate derivatives, including juice, extracts, and phytochemicals, have shown preventive and therapeutic effects on colon, liver, lung, prostate, and skin cancers (13).
Studies have also investigated the concurrent effects of exercise and pomegranate juice (PJ). For example, Akbarpour et al. found that 8 weeks of aerobic exercise and PJ consumption significantly decreased mir-155 in men with prostate cancer, with the greatest reduction observed in the group that combined aerobic exercise with PJ consumption (11). Overall, regular physical activity appears to reduce mortality in cancer patients (7). However, its molecular mechanisms are not well understood.
Additionally, despite the growing research and scientific advances in understanding pomegranate and its effects on oxidative factors in diseases, including diabetes and cancer, the therapeutic properties of this fruit have been understudied (11). Therefore, this study seeks to answer whether a period of high-intensity interval training (HIIT) combined with PJ consumption has a significant effect on the G-CSF signaling pathways in mice with colon cancer.
2. Objectives
Therefore, this study seeks to answer whether a period of HIIT combined with PJ consumption has a significant effect on the downstream pathway of G-CSF in laboratory mice with colon cancer.
3. Methods
3.1. Animal Procurement and Maintenance
This experimental study employed a post-test design with control groups. Initially, 30 male BALB/c mice (weight range: 18 - 22 g, age: 8 - 10 weeks) were obtained from the Pasteur Institute of Iran and transferred to the laboratory. The animals were maintained for 1 week without any intervention to allow acclimatization to their new environment. Throughout the study, mice were housed under standardized conditions, including a Light/dark cycle: 12-hour light/12-hour dark, Controlled temperature: 22 - 24°C, Relative humidity: 55%, Quiet environment free from noise disturbances. The animals were housed in washable polycarbonate cages. Sterile wood shavings were used as bedding material to absorb cage moisture. All ethical principles for laboratory animal research were followed in accordance with the Ethics Committee for Biomedical Research and the Helsinki Declaration.
3.2. Cell Line and Colon Cancer Induction
CT26 cells were initially obtained from the Pasteur Institute of Iran. When the cells formed a complete monolayer at the bottom of the flask, they were trypsinized and washed 3 times. Cell count and viability were determined using the trypan blue exclusion method under a standard microscope with a Neubauer chamber. After sufficient cell proliferation, 24 animals were injected subcutaneously in the right flank with 5 × 105 viable cells suspended in 150 μL of Hanks' solution. Following the confirmation of CRC induction, the diseased mice were randomly divided into 4 groups, including cancer control group (CRC) (n = 6), PJ supplement group (n = 6), Exercise training group (HIIT) (n = 6), Combined PJ and exercise group (HIIT + PJ) (n = 6).
3.3. High-Intensity Interval Training Protocol
Following familiarization, the maximum running speed of laboratory mice was measured in a dedicated session. The maximum speed assessment protocol was as follows: Mice initially performed a 3-minute warm-up at 10 meters per minute on a 5-degree incline. Subsequently, the speed was increased by 3.3 meters per minute every minute on a 10-degree incline. This progression continued until the mice reached exhaustion. Exhaustion was defined as the point, at which animals hit the end of the treadmill 3 consecutive times within 1 minute. After reaching exhaustion, mice were cooled down for 3 minutes at speeds below 10 meters per minute before being returned to their cages (14) (Table 1).
| Stages | Speed (m.min-1) | Incline (Degree Slope) | Duration (min) |
|---|---|---|---|
| Warm-up | 10 | 5 | 3 |
| 1 | 13 | 10 | 1 |
| 2 | 16.6 | 10 | 1 |
| 3 | 19.9 | 10 | 1 |
| 4 | 23,2 | 10 | 1 |
| 5 | 26.5 | 10 | 1 |
| 6 | 29.8 | 10 | 1 |
| * | … | 10 | 1 |
| Cool-down | < 10 | < 5 | 3 |
a Exhaustion was defined as the point where mice refused to run, stopped 3 times in a row, or remained more than 10 seconds on a small resting area at the extremity of the running belt.
The HIIT protocol was implemented through a carefully structured progression over 8 weeks. During the initial 2 weeks, rats participated in 5 weekly training sessions, each lasting 30 minutes. Every session commenced with a comprehensive 10-minute warm-up phase performed at an intensity below 50% of their maximum speed. The main workout consisted of alternating between high and low-intensity intervals, specifically 5 sets of 1-minute high-intensity bouts exceeding 75% of maximum speed, interspersed with 4 sets of 2-minute low-intensity recovery periods at less than 50% of maximum speed. Each session concluded with a 10-minute cool-down period to ensure proper recovery. From week 2 onwards, the protocol was modified and intensified. The warm-up phase was condensed to 3 minutes while maintaining the intensity below 50% of maximum speed. The main workout structure was adjusted to incorporate more high-intensity intervals, beginning with 8 one-minute sets at 85% to 90% of maximum speed, alternating with 7 one-minute low-intensity recovery intervals at less than 50% of maximum speed (Table 2). To ensure progressive overload, 2 key modifications were implemented: Firstly, during weeks 6 through 8, a 15-degree incline was introduced to increase workout intensity. Secondly, the number of high-intensity intervals was progressively increased, starting from 8 sets and reaching 16 sets by the 8th week, while maintaining the same intensity parameters (14).
| Weeks | Warm-up - Cool-down | HIIT Protocol | |||
|---|---|---|---|---|---|
| Max Speed (%) | Duration (min) | Frequency (N) | Intensity (%) | Incline (Grade) | |
| 1 | > 50 | 10 | 5 | 75 < | 0 |
| 2 | > 50 | 10 | 5 | 75 < | 0 |
| 3 | > 50 | 3 | 8 | 85 < | 10 |
| 4 | > 50 | 3 | 10 | 85 < | 10 |
| 5 | > 50 | 3 | 12 | 85 < | 12 |
| 6 | > 50 | 3 | 14 | 90 < | 12 |
| 7 | > 50 | 3 | 16 | 90 < | 15 |
| 8 | > 50 | 3 | 16 | 90 < | 15 |
Abbreviation: HIIT, high-intensity interval training.
3.4. Pomegranate Juice Supplementation
After washing the fruit, the pomegranate peel was removed. The edible portions (exclusively pomegranate seeds) were juiced using a blender. The prepared juice was filtered through paper filtration; subsequently, the filtered liquid was centrifuged and maintained in a stable environment to separate the clear supernatant. The clear PJ was, then, stored at -20°C. Given the sensitive nature of PJ and its low stability, juice was prepared every 3 days, with juice extraction repeated for the subsequent 3-day periods. Daily, 5% PJ was combined with drinking water for laboratory mice consumption (15).
3.5. Dissection and Sampling
Forty-eight hours following the final exercise session and after a 12-hour fasting state, laboratory mice were anesthetized using ketamine (55 mg/kg) and xylazine (15 mg/kg). Blood was subsequently collected directly from the heart tissue using a 2 mL syringe. The intestinal tissue was carefully extracted from the upper colon region and stored at -70°C for subsequent analysis.
3.6. Gene Expression Measurement Technique
Gene Expression Measurement G-CSFR, JAK2, STAT3, MAPK, and ERK were quantified using quantitative Real-Time PCR. A small quantity of homogenized intestinal tissue was initially used for RNA synthesis. RNA synthesis was performed using a specialized extraction kit from Qiagen, Germany. RNA purity was assessed via spectrophotometry at 260 nanometer wavelengths. Upon confirming acceptable concentration, samples were processed for cDNA synthesis using an extraction kit from ROCHE, Germany. Samples were, then, combined with pre-designed primers previously validated on PUBMED. These samples were placed in a real-time PCR device (step one, manufactured in Italy). Upon completion of various temperature and time cycles, variable values were evaluated alongside internal control genes based on the threshold cycle (Ct). Data quantification was ultimately performed using the 2-ΔΔCt formula (Table 3).
| Gene Names | Product Size |
|---|---|
| TBP | 147 |
| Forward: 5’-GCGGGGTCATGAAATCCAGT-3’ | |
| Reverse: 5’-AGTGATGTGGGGACAAAACGA-3’ | |
| MAPK1 | 249 |
| Forward: 5’-ACCCAAGTGATGAGCCCATTG-3’ | |
| Reverse: 5’-TCAATGGAAGGGGACAAACTGAA-3’ | |
| ERK | 202 |
| Forward: 5’-CACTGGCTTTCTGACGGAGT-3’ | |
| Reverse: 5’-TGGGGAACCCAAGATACCTAGA-3’ | |
| STAT3 | 286 |
| Forward: 5’-TCAGCGAGAGCAGCAAAGAA-3’ | |
| Reverse: 5’-TACGGGGCAGCACTACCT-3’ | |
| G-CSF-R | 136 |
| Forward: 5’-AAGTTCACCAGGCAGGTAAG-3’ | |
| Reverse: 5’-GGGGGTGAAATCTCGATGTG-3’ | |
| JAK2 | 261 |
| Forward: 5’-GTGTCGCCGGCCAATGT-3’ | |
| Reverse: 5’-CCACAAGCTTTAGAAGCAGCC-3’ |
Abbreviations: MAPK, mitogen-activated protein kinase; JAK2, Janus kinase 2.
3.7. Statistical Analysis Methods
Data were presented using mean and standard deviation. The distribution of data was assessed using the Shapiro-Wilk test. An independent t test was employed to examine differences between healthy control (HC) and patient control groups. To evaluate exercise effects, supplementation, and exercise + supplementation interaction, one-way ANOVA with Bonferroni post-hoc test was utilized. All research data were analyzed using SPSS software (version 26). Statistical significance was set at P ≤ 0.05.
4. Results
4.1. G-CSFR
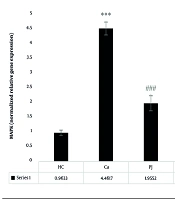
Analysis of the G-CSFR revealed significant differences between the patient control group and the supplement, exercise, and exercise + supplement groups. A notable significant difference was also observed between the supplement and exercise + supplement groups. Interestingly, no statistically significant variations were found between the supplement and exercise groups, nor between the exercise and exercise + supplement groups (Figure 1).
G-CSFR gene expression levels in the colon tissue of laboratory mice across the study groups. *** (P = 0.001): A significant increase compared to the healthy control (HC) group. P = 0.001: A significant decrease compared to the Ca group. $ (P = 0.05): A significant decrease compared to the pomegranate juice (PJ) group. ### (P = 0.001) indicates a significant decrease compared to the Ca group.
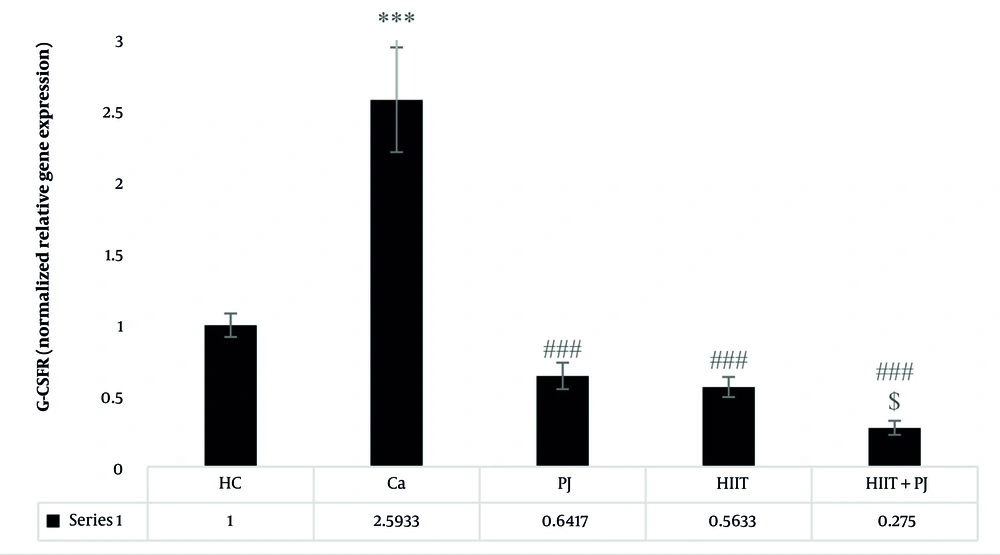
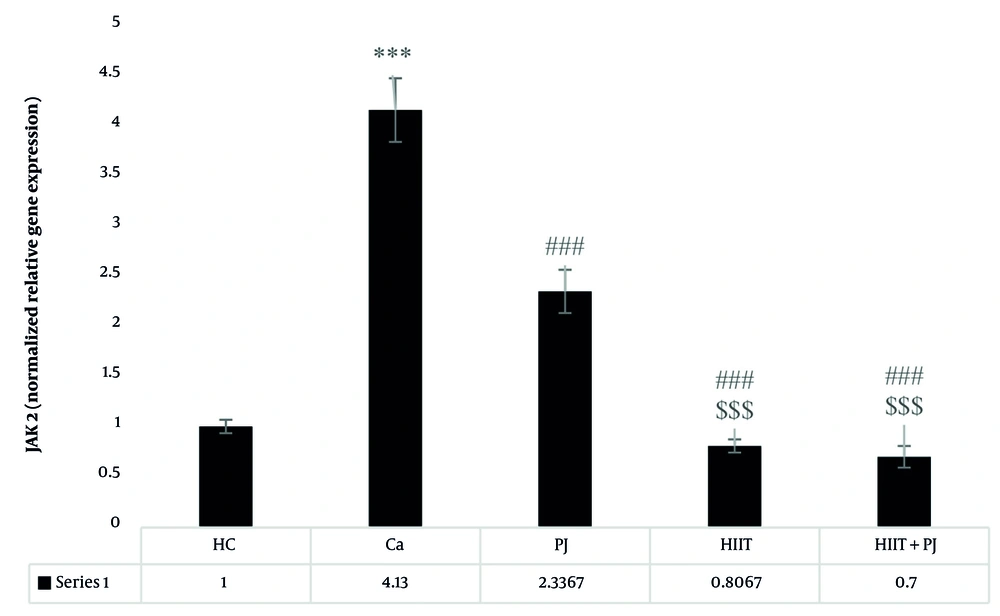
4.2. Janus Kinase 2
For the JAK2, significant differences were detected between the patient control group and the supplement, exercise, and exercise + supplement groups. Additionally, significant differences emerged between the supplement and exercise groups, as well as between the supplement and exercise + supplement groups. However, no significant difference was observed between the exercise and exercise + supplement groups (Figure 2).
Janus kinase 2 (JAK2) gene expression levels in the colon tissue of laboratory mice across the study groups. *** (P = 0.001): A significant increase compared to the healthy control (HC) group. P = 0.001: A significant decrease compared to the Ca group. $$$ (P = 0.001): A significant decrease compared to the PJ group. ### (P = 0.001) indicates a significant decrease compared to the Ca group.
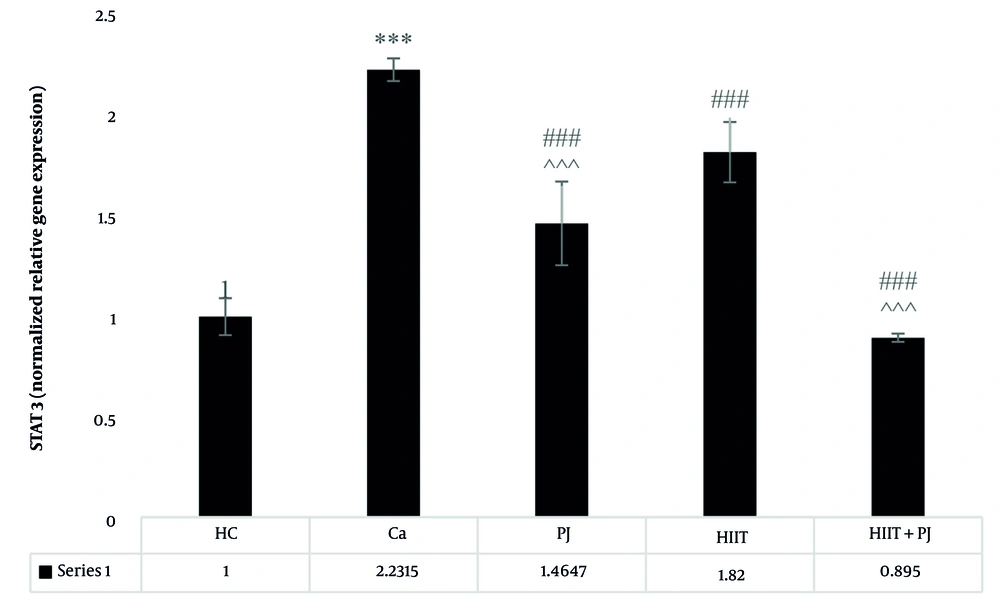
4.3. STAT3
The STAT3 demonstrated a complex pattern of significant differences. Statistically significant variations were found between the patient control group and the supplement, exercise, and exercise + supplement groups. Furthermore, significant differences were observed between the supplement and exercise groups, between the supplement and exercise + supplement groups, and notably, between the exercise and exercise + supplement groups (Figure 3).
STAT3 gene expression levels in the colon tissue of laboratory mice across the study groups. *** (P = 0.001): A significant increase compared to the healthy control (HC) group. P = 0.001: A significant decrease compared to the Ca group. ^^^ (P = 0.001): A significant decrease compared to the high-intensity interval training (HIIT) group. ### (P = 0.001) indicates a significant decrease compared to the Ca group.
4.4. Mitogen-Activated Protein Kinase
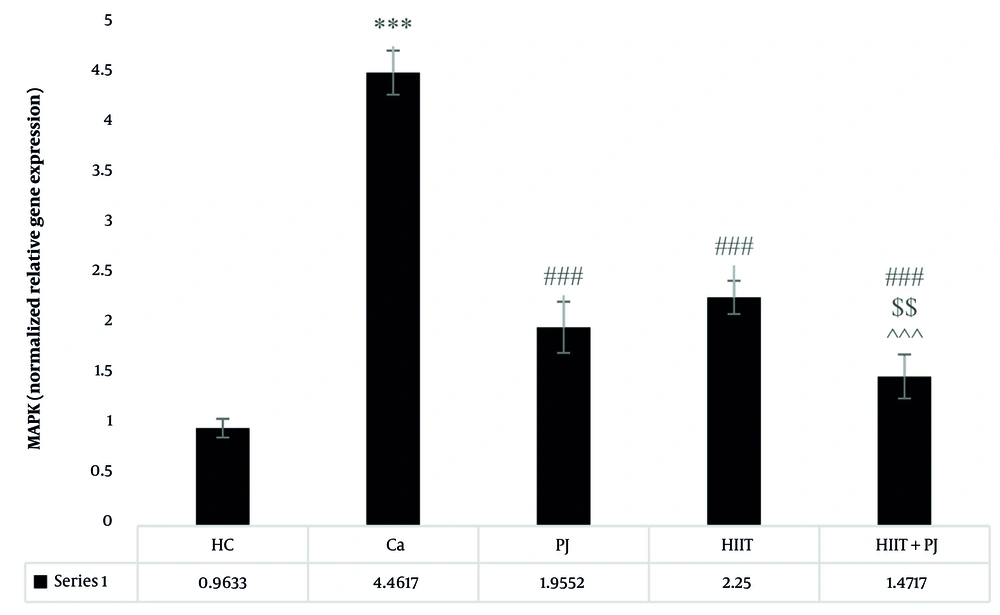
Examining the MAPK, significant differences were identified between the patient control group and the supplement, exercise, and exercise + supplement groups. Significant differences were also found between the supplement and exercise + supplement groups, as well as between the exercise and exercise + supplement groups. Notably, no significant differences were detected between the supplement and exercise groups (Figure 4).
Mitogen-activated protein kinase (MAPK) gene expression levels in the colon tissue of laboratory mice across the study groups. *** (P = 0.001): A significant increase compared to the healthy control (HC) group. P = 0.001: A significant decrease compared to the Ca group. $$ (P = 0.01): A significant decrease compared to the pomegranate juice (PJ) group. ^^^ (P = 0.001): A significant decrease compared to the high-intensity interval training (HIIT) group. ### (P = 0.001) indicates a significant decrease compared to the Ca group.
4.5. ERK
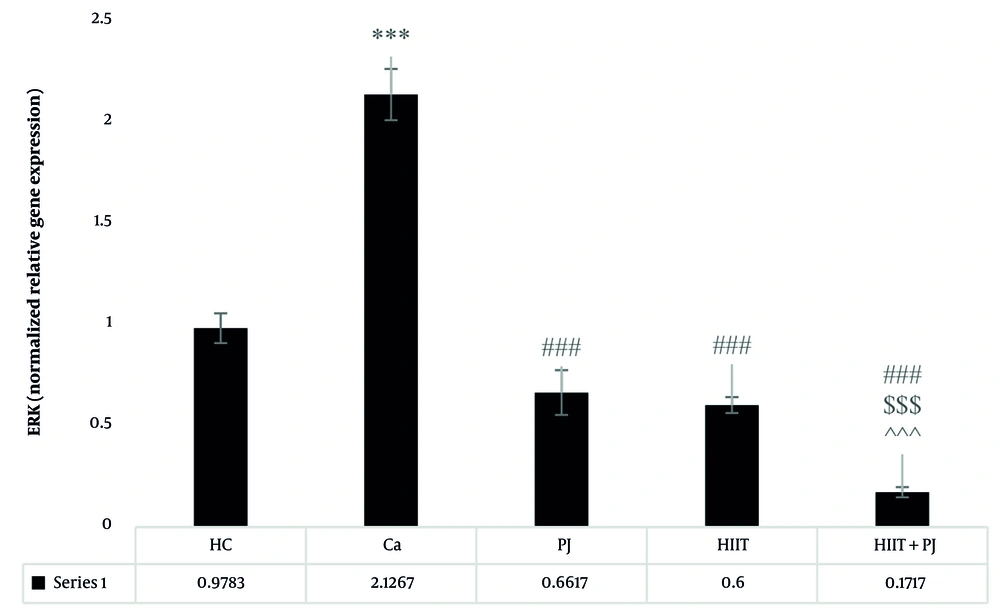
The ERK analysis revealed significant differences between the patient control group and the supplement, exercise, and exercise + supplement groups. Significant variations were also observed between the supplement and exercise + supplement groups, and between the exercise and exercise + supplement groups. Consistent with the MAPK, no significant differences were found between the supplement and exercise groups (Figure 5).
ERK gene expression levels in the colon tissue of laboratory mice across the study groups. *** (P = 0.001): A significant increase compared to the healthy control (HC) group. P = 0.001: A significant decrease compared to the Ca group. $$$ (P = 0.001): A significant decrease compared to the pomegranate juice (PJ) group. ^^^ (P = 0.001): A significant decrease compared to the high-intensity interval training (HIIT) group. ### (P = 0.001) indicates a significant decrease compared to the Ca group.
5. Discussion
The results demonstrated that the induction of CRC leads to increased expression of G-CSFR, JAK2, STAT3, MAPK, and ERK genes in the intestinal tissue of laboratory mice. It appears that hematopoietic growth factors or CSF are the primary growth stimulants in cells, and their expression becomes progressively dysregulated following CRC. Studies indicate that when G-CSF binds to its receptor, G-CSFR, it triggers the release and migration of stem cells under both physiological and pathological conditions, enhancing these cells' ability to differentiate and proliferate into intestinal cells (16). Colony-stimulating factor subsequently activates pathways such as JAKs and STATs, with JAK2 and STAT3 being identified as the most significant in CRC development. Researchers believe that STAT3 protein creates a negative feedback control through SOCS transcription regulation, leading to increased tumorigenesis (17). Furthermore, researchers have reported that JAK2 proteins, besides activating STAT3, can trigger the MAPK pathway regulated by the RAS gene product, which leads to increased reactive oxygen species and enhanced apoptosis in healthy intestinal cells (18). Additionally, ERK activation following MAPK signaling pathway activation appears to activate various pro-inflammatory cytokines, including IL-6, IL-8, TNF-α, and TGF-β (19). Consequently, the ERK1/2 pathway primarily responds to growth factor stimulation, potentially contributing to the increased proliferation of cancer cells in the intestine (20).
The results indicated that HIIT training reduces the expression of G-CSFR, JAK2, STAT3, MAPK, and ERK genes in the intestinal tissue of laboratory mice with CRC. Regular physical activity appears beneficial in cancer conditions through improved energy substrate metabolism, reduced inflammatory factors, and enhanced antioxidant system function (21). Physical activities and exercise release myokines that increase anti-tumor effects and improve micronutrient absorption in CRC patients (2). Researchers have demonstrated that physical activity in CRC patients can effectively improve metabolism and treatment progression (22). They reported that acute and chronic interval training could reduce inflammatory factors, decrease the number of cancer cells in the colon, increase serum levels of IL-6 and IL-8, and reduce tumor necrosis factor-alpha (TNF-α), ultimately leading to tumor growth arrest (23). Researchers believe that regular exercise, with its ability to induce specific physiological stimuli that may mobilize progenitor cells, can influence cellular biological processes (24). In a study, researchers reported that a single session of moderate-intensity activity increases serum G-CSF levels in trained individuals, stimulating the recruitment of stem cells from bone marrow (25). It should be noted that this effect was evaluated in healthy individuals; however, a significant point is that single-session exercise can also lead to increased stimulating factors. Therefore, this study was not consistent with the present study. Overall, studies examining the effect of exercise adaptation on G-CSF levels and stem cells are limited, and even these few studies have reported contradictory results. On the other hand, the protective role during acute injuries and adaptation to HIIT due to greater hypoxia compared to other exercises has been accepted. While acute exercises increase stem cells for hematopoietic cell production in response to hypoxia, in cancer, due to metastases and their activity, this process leads to excessive cell proliferation and tumor formation. Additionally, performing these types of exercises in less time shows more significant results (26). Researchers believe that continuous physical activities are a facilitating factor in suppressing inflammatory diseases. It can lead to reduced visceral fat mass, decreased pro-inflammatory adipokine release, and reduced neutrophil and macrophage levels in the tumor tissue microenvironment (27). Researchers also reported that physical activity could direct macrophage polarization in adipose tissue toward an anti-tumor phenotype, consequently reducing overall body inflammation and tumor volume (27). Regarding the effect of exercise on the MAPK and ERK pathways, there appears to be some contradiction. Some studies have shown that physical activity increases ERK gene and protein expression through growth factor secretion, muscle tension, oxidants, and pH reduction (28). However, these studies were conducted in non-cancer samples and serum or other tissues; therefore, these factors may explain the contradictory results. In another study, researchers reported that ERK is activated immediately after activity (28), which contradicts the present study. Nevertheless, Williamson et al. reported a significant decrease in MAPK following a single session of knee extension resistance training (29 contractions at 70% maximum) in elderly men (29). Changes from aerobic exercise may be partly due to the regulation of MAPKs that are affected by ROS regardless of their source. In other words, the reduction in oxidative stress from long-term exercise leads to decreased ERK½ levels with reduced P38, resulting in reduced molecules involved in apoptosis and oxidative stress (19). Despite limited information regarding the effect of exercise on downstream colony stimulants, Standard et al. reported that after 10 weeks of physical activity, RAS-MAPK signaling expression decreases, leading to reduced cancer incidence associated with weight loss (30).
Results indicated that PJ supplement reduces the expression of G-CSFR, JAK2, STAT3, MAPK, and ERK genes in the intestinal tissue of laboratory mice with colon cancer. Synthetic antioxidants primarily contain phenolic compounds, and pomegranate is one of the richest sources. Components such as vitamin C, β-carotene, catechins, anthocyanins, butylated hydroxytoluene, butylated hydroxy, tert-butyl hydroquinone, and gallates are the main constituents of pomegranate that possess anti-cancer properties. Researchers have demonstrated that pomegranate and its constituents, acting as an antioxidant, initially inhibit tumor formation by suppressing free radicals and nuclear transcription factor kappa B (NF-κB). Furthermore, through its antioxidant effects, it inhibits apoptosis, activates deacetylases, and increases AMPK, leading to enhanced nuclear transcription and improved cellular metabolism (31). Although comprehensive information regarding PJ's effects on intestinal tissue is limited, evidence suggests that PJ supplement improves oxidative stress and inflammatory factors by increasing adiponectin in visceral adipose tissue while reducing TNF-α, IL-1β, and other inflammatory factors (32). In another study, researchers reported that pomegranate activates the AMPK pathway, leading to increased UCP1/2 and PRDM16, ultimately enhancing thermogenesis and cellular protective proteins (33). Additional research demonstrated that pomegranate flower extract reduces LDL, VLDL, atherosclerotic plaque marker proteins, and cardiac damage markers in SR-BI/apoE double KO mice (34). As mentioned, the lipolytic mechanisms of PJ are not yet fully understood. However, most studies point to its potent effects on improving fat metabolism in adipose tissue and liver pathways, ultimately enhancing blood lipid profiles. Nevertheless, information regarding the downstream G-CSF gene expression pathway in intestinal tissue and colon cancer conditions remains limited; therefore, further research in this area is needed. One of the innovative aspects of the present study was the use of a plant-based antioxidant (PJ) and its effects on the aforementioned pathway in the intestinal tissue of laboratory mice with colon cancer.
High-intensity interval training exercise and PJ have a synergistic effect on reducing the expression of G-CSFR, JAK2, MAPK, and ERK genes in the intestinal tissue of laboratory mice with colon cancer. Despite extensive research, no study was found examining the signaling pathway of this study. However, researchers have shown that consuming PJ supplement alongside acute exercise leads to improved oxygen intake, nitrate levels, and athletic performance following an exhaustive cycling session (14). In another study, researchers demonstrated that PJ consumption has a positive effect on athletic performance and post-exercise recovery. Researchers in a study showed that 8 weeks of aerobic exercise combined with PJ supplement led to decreased expression of miR-21, miR-155, and increased P53 in men with prostate cancer (11). It has also been reported that aerobic exercise combined with PJ supplement improves antioxidant system function in women with breast cancer (35). It has been reported that aerobic exercise and PJ consumption led to improvement in anthropometric indices and liver enzymes, and lipid metabolism in individuals with type 2 diabetes (36). Based on this, it appears that exercise and PJ supplement reinforce each other's effects through similar pathways and are effective in inhibiting the downstream G-CSFR, JAK2/STAT3 pathway, leading to inhibition of tumorigenesis pathways in intestinal cancer tissue (32, 33).
5.1. Conclusions
Overall, the results showed that although the induction of intestinal cancer increases the expression of G-CSFR, JAK2, STAT3, MAPK, and ERK genes in the intestinal tissue of laboratory mice, both HIIT exercise and PJ individually and synergistically through similar antioxidant and anti-inflammatory pathways can reduce the expression of G-CSFR, JAK2, STAT3, MAPK, and ERK genes in the intestinal tissue of laboratory mice with colon cancer.
5.2. Limitations
The present study acknowledges certain limitations, particularly the non-measurement of oxidative stress and inflammatory factors in intestinal tissue, which are crucial in cancer progression. Additionally, the study did not assess catecholamines and myokines, which play significant roles in metabolic processes.
5.3. Suggestions
Future research should incorporate evaluations of oxidative stress and inflammatory mechanisms to provide a more comprehensive understanding. It is also recommended that subsequent studies explore muscle-related pathways and the changes in catecholamines following exercise, particularly their effects on intestinal tissue.