1. Background
Disease burden is estimated as cause-specific disability-adjusted life years (DALYs) (1). In 2020, there were more than 2.3 million patients with breast cancer and 685000 cancer related death worldwide. Most cancer DALYs are for years of life lost (97%), and only less than 3% came from years lived with disability (2, 3). Breast, lung, and prostate are the most frequent cancers worldwide (1). Solid tumors are more prevalent among malignant diseases, and the practical step for them is primary management (surgery and tumor removal). Assessing locoregional lymph nodes is necessary as a prognostic factor and sometimes has a therapeutic effect (4). Each tumor has its tumor-draining lymph node(s) (TDLNs) (5, 6). The lymphatic fluid which drains away from the primary tumor may contain tumor cells and/or tumor-derived factors. Tumor cells that go to the lymphatic system can also reach the blood circulation and disseminate to other distant organs to form metastasis. The TDLN may contain metastatic cells, and analysis of it is critical to the clinical staging system in the metastatic process. The surgical removal of TDLN is now widely used in clinics to determine future treatment (7). Although the sentinel lymph node (SLN) involvement is an important prognostic factor, complete lymph node dissection (CLND) does not promote survival. Clinical trials reported little or no benefit of CLND on survival, even after several decades of follow-up (8-15). The role of TDLNs in tumor growth and dissemination is poorly understood, and therefore, the limitation of lymphadenectomy in solid tumors needs further investigation. For example, in Western countries, limited lymph node dissection (LND) for gastric cancer or doing it in specialized centers is recommended without beneficiary of extensive LND (16, 17).
2. Objectives
This study investigated local management with and without LND on the overall survival (OS) of mice inducted with 4T1 breast cancer to determine the role of lymphadenectomy in cancer surgery.
3. Methods
3.1. Ethics
The project was performed according to the ethical principles, standards for conducting medical research, and national norms in Iran. It was evaluated by the Research Ethics Committees of Shahid Beheshti University of Medical Sciences (Cancer Research Center), with approval ID: "IR.SBMU.CRC.REC.1400.044". This article is reported according to the ARRIVE v2.0 guideline (18).
3.2. Cell Line and Culture Conditions
A mouse mammary tumor cell line (4T1) was obtained from Pasteur Institute (Iran, Tehran). Cells were cultured in RPMI medium supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum (FBS) and incubated at 37°C in a 5% CO2 and 70% humidity atmosphere. Following appropriate growth, the cultured cells were harvested using trypsin (Gibco Co., Germany) to detach the cells from cell culture flask surfaces. Then, the cells from the cell culture were washed twice with phosphate buffer saline (PBS). Finally, the number of alive or dead cells was counted using a cell counting microscopic slide. Differential diagnosis between dead and alive cells was performed using Trypan blue staining.
3.3. Animals
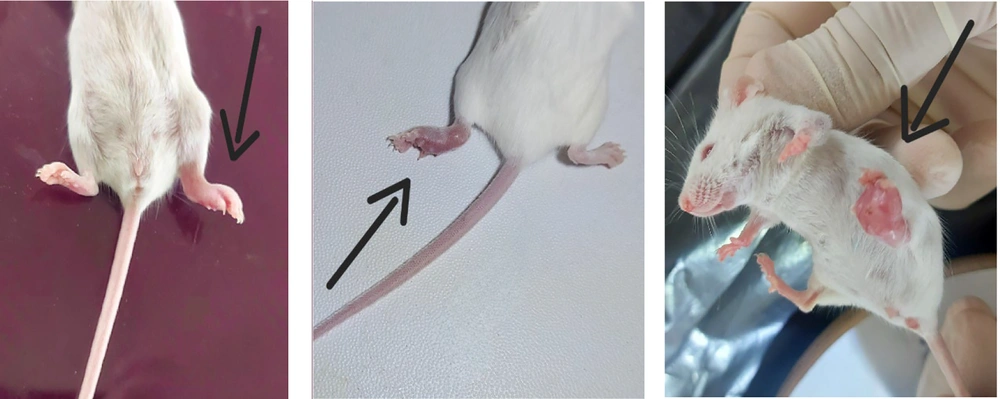
Female BALB/c in-breed mice were purchased from the Pasteur Institute (Iran, Tehran). All mice recruited in this study were 8 - 10 weeks old and were kept under normal conditions in the animal care facility (Isfahan University of Medical Science). They were kept in a 12-hour light-dark cycle and fed with commercial mouse diet food. Animals were carefully monitored throughout the experiment for signs of pain and severe stress, such as decreased eating or drinking, reduced activities, and weight loss. An insulin syringe was used to inject 0.1ml PBS buffer containing 106 4T1 cells. The cells were injected subcutaneously into the left foot of each mouse. Light pressure was applied to the foot as the needle was withdrawn to prevent back-flow.
3.4. Grouping
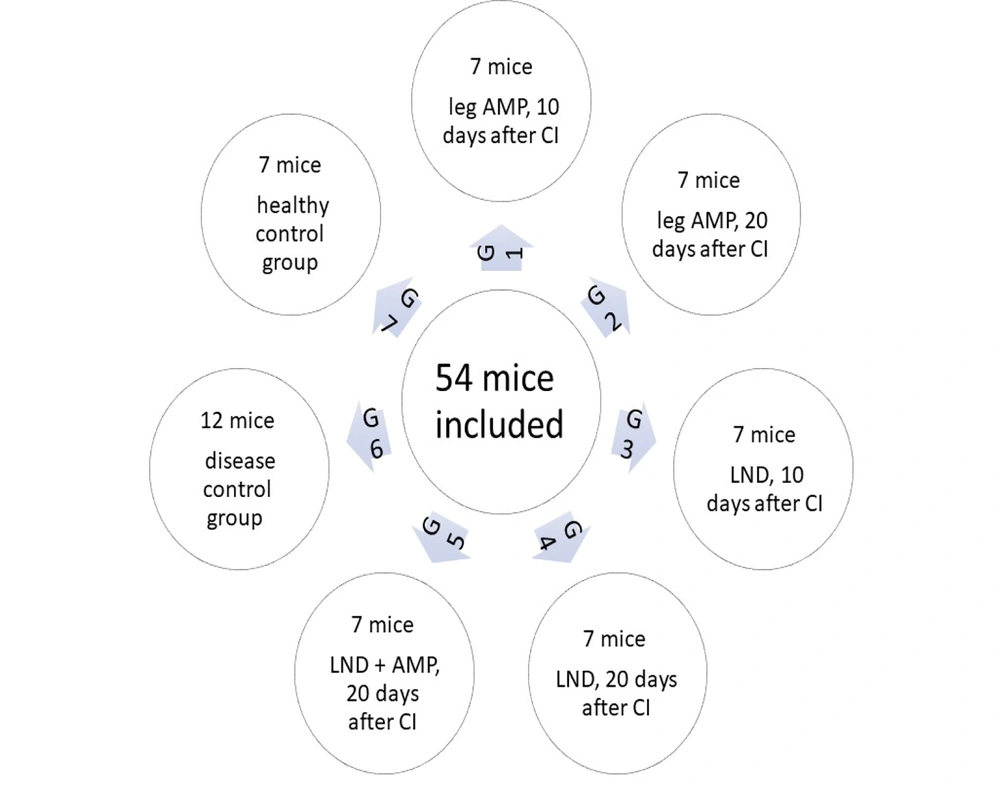
In this study, 54 mice were used and divided into seven groups. Every group had seven mice except group 6, which had 12. This sample size was determined based on previous studies assessing survival outcomes in murine cancer models. The number of animals per group was selected to ensure statistical power while adhering to ethical guidelines for animal research. All of them were injected with tumor cells except the last group. In the first (early local management) and second (delayed local management) groups, the tumoral legs were subjected to local management (because we couldn't excise the tumor, we amputated the leg above the knee) 10 and 20 days after cell injection, respectively. In the third and fourth groups, inguinal LND in the tumoral side was done on days 10 and 20, respectively. In the fifth group, on day 20, the combination of inguinal LND and above-knee amputation (AMP) was performed after cell injection. Mice in the sixth group were considered a control group and only injected with tumor cells (diseased control group). The seventh group was left intact without any intervention as the healthy control group.
3.5. Follow-up
The presence of solid tumors was checked every day, and following detection, the tumor volume of all animals was measured every three days up to the end of the study (Figure 1). All the mice were then followed for 120 days, and after that, they were sacrificed. The timeline of the study is shown in Figure 2.
Study timeline: 54 mice were included in 7 groups (G). Groups 1 - 6 were injected with breast cancer cells. G1: Seven mice; subjected to leg amputation (AMP) 10 days after cancer injection. G2: Seven mice; subjected to leg AMP 20 days after cancer injection. G3: Seven mice; subjected to lymph node dissection (LND) 10 days after cancer injection. G4: Seven mice; subjected to LND 20 days after cancer injection. G5: Seven mice; subjected to LND + leg AMP 20 days after cancer injection G6: Twelve mice; no surgical intervention as a disease control group. G7: Seven mice; left intact as the healthy control group. (Abbreviation: CI, cancer injection.)
3.6. Surgical Procedures and Lymphadenectomy
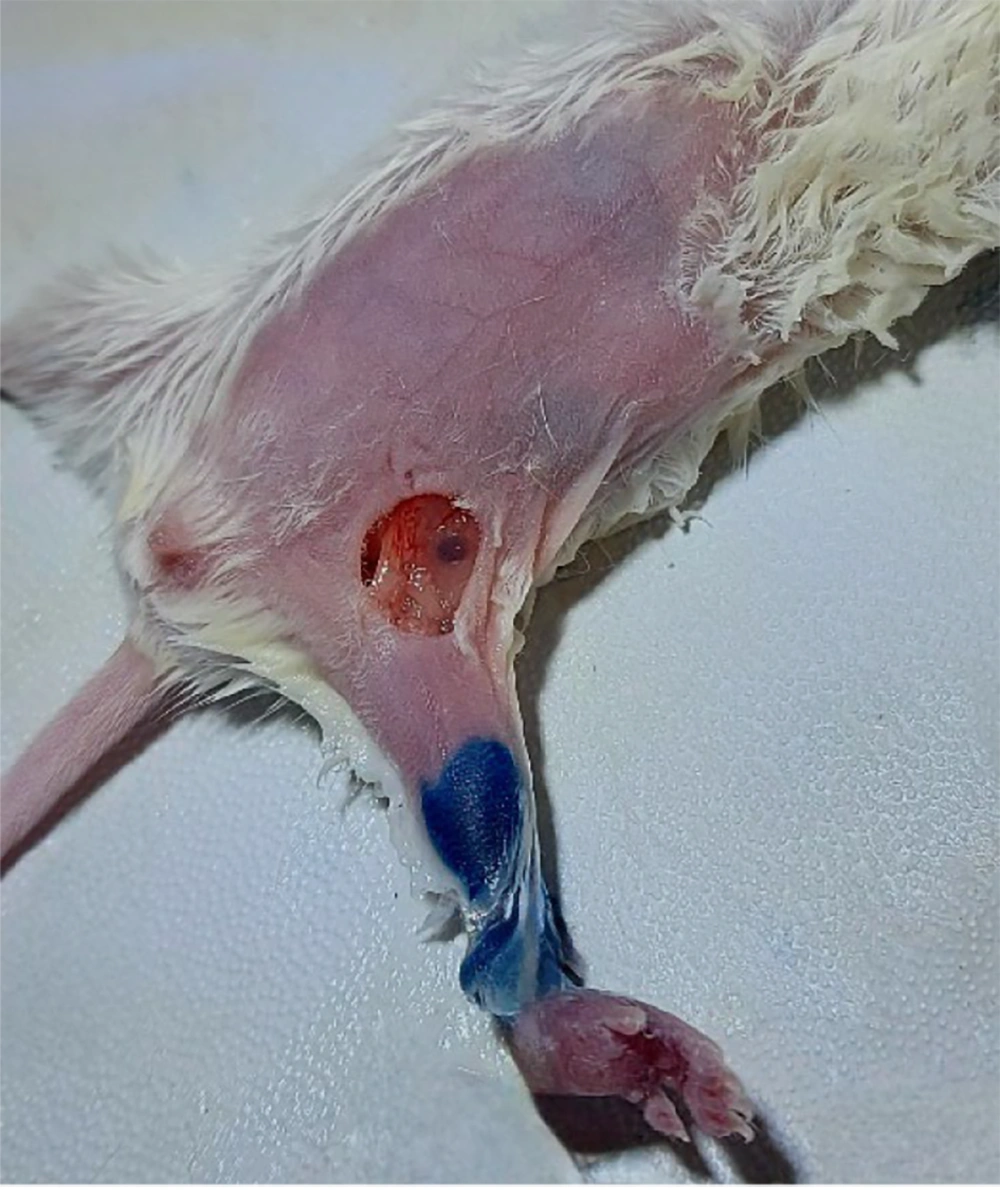
Before surgical interventions, mice were anesthetized by intraperitoneal injection of Ketamine. The lack of toe pinch withdrawal reflexes confirmed the success of anesthesia. The leg was cut above the knee with scissors for AMP, and the stump was suture ligated. For LND, the inguinal hair was removed by hair remover cream, and then 0.1 ml methylene blue was injected into the tumoral foot (to prove the exact location of the lymph node). The inguinal region was explored, all inguinal lymph nodes were dissected (Figure 3), and the skin was sutured. No prophylactic antibiotic was injected. The liver and lungs were removed for every mouse that died or survived for 120 days. After AMP (Figure 4), The LND, and autopsy, the specimens were collected in 10% formalin and sent to the pathology laboratory.
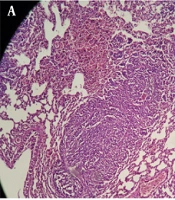
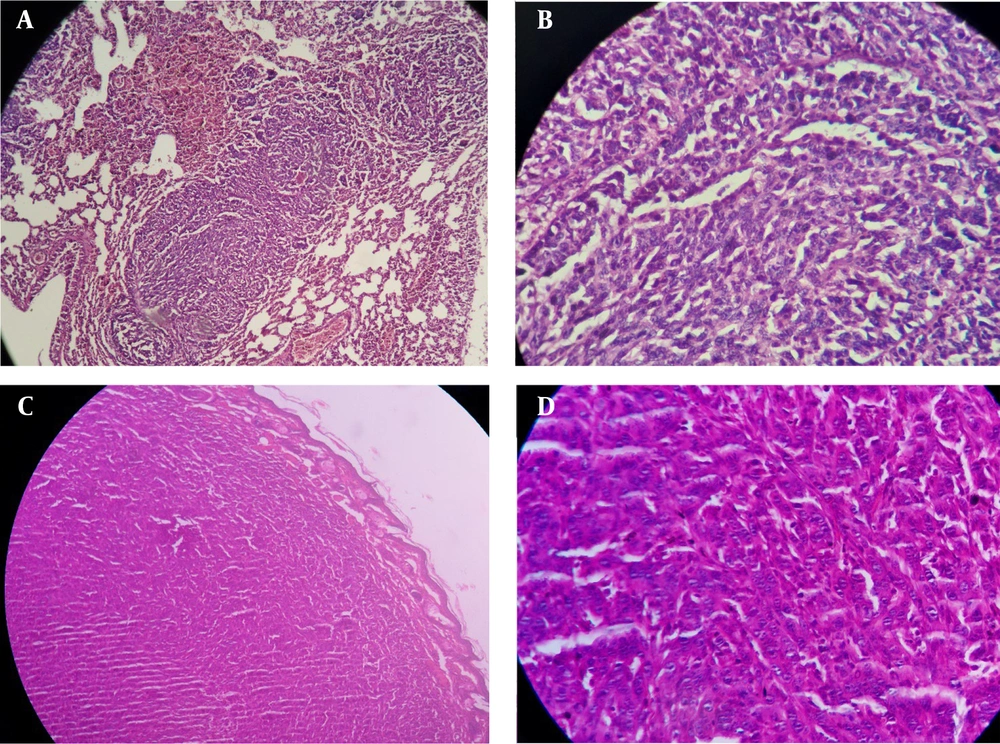
Amputated foot and lung metastasis in mice injected with 4T1 breast cancer cells. A, Lung biopsy; sections show neoplastic proliferation of epithelial cells as a solid pattern (low power field); B, neoplastic cells with marked pleomorphism (in lung biopsy), hyperchromatic nuclei, and prominent nucleoli (high power field); C, foot biopsy; sections show neoplastic proliferation of epithelial cells as a solid pattern (low power field); and D, neoplastic cells with marked pleomorphism (in foot biopsy), hyperchromatic nuclei, and prominent nucleoli (high power field).
3.7. Preparation of Pathology Slides
After processing the samples in a Tissue processor, they were molded inside paraffin blocks, and slides were prepared after cutting with a microtome. Hematoxylin-eosin staining was performed on the slides.
3.8. Statistical Analyzes
All quantitative data were reported as mean ± SEM. Nominal and rank data are reported based on percentage. Analysis of survival distribution was performed using the Kaplan-Meier test as log-rank pairwise. The Metastasis Index of cancer cells between different studied groups was done using the chi-square test. All statistical analyses were done using SPSS software version 21 (IBM® SPSS® Statistics, USA), and a P-value less than 0.05 was significant. A pairwise log-rank test was performed to analyze the survival distribution among the studied groups.
4. Results
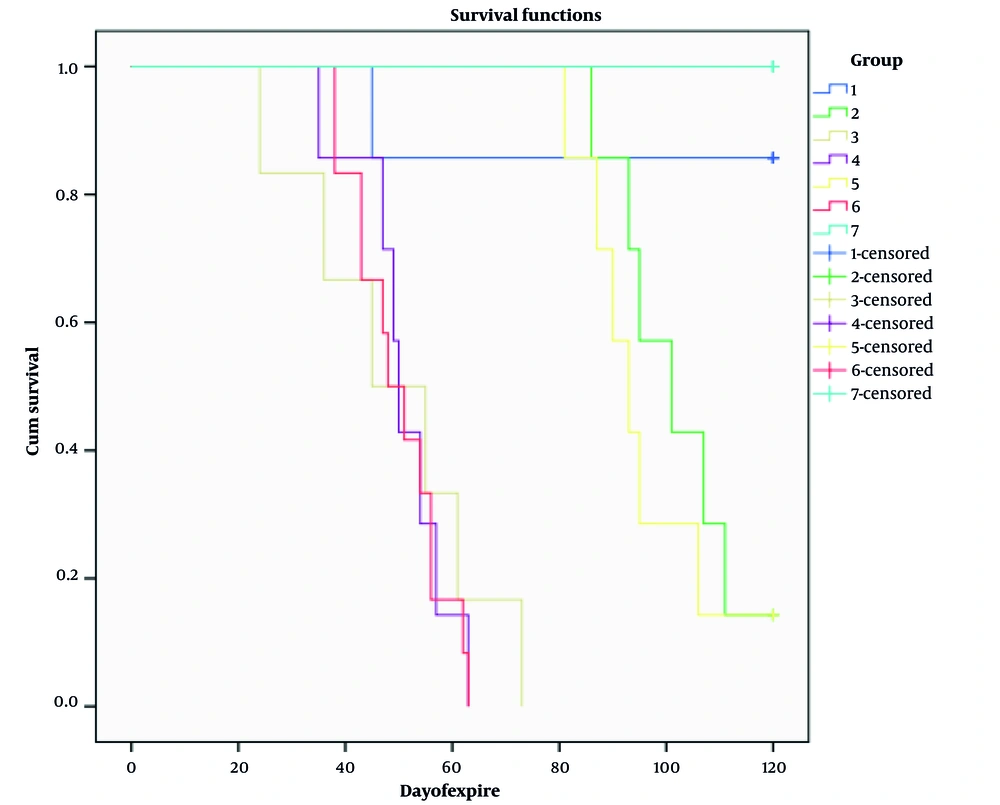
All mice in group 1 (early AMP), except one, survived 120 days. When they were sacrificed, there was no sign of metastasis; all were healthy. Also, in the dead mouse, there was no sign of metastasis (liver and lung). In group 2 (delay AMP), the first mortality happened on day 86. One mouse in this group had metastasis (Figure 4). In group 3 (early LND), one mouse died on the day of the procedure, and it was due to a technical error. Five of the six remaining mice had metastasis (liver and lung). After LND, no lymph nodes were involved by the tumor. In group 4 (delay LND), five of seven mice had metastasis, and lymph nodes were detected in all of them, but the tumor did not involve them. In group 5 (AMP + LND), two mice had metastasis, and no lymph nodes were involved in the tumor. In group 6 (diseased control), there were 12 mice, and 10 had metastasis. In group 7 (healthy control), all survived for 120 days. Survival time in group 1 (χ2 = 11.6, P = 0.001), group 2 (χ2 = 16.73, P < 0.0005), and group 5 (χ2 = 16.73, P < 0.0005) was significantly higher than that of group 6. While in groups 3 (χ2 = 0.15, P = 0.69) and 4 (χ2 = 0.06, P = 0.79), no significant changes were observed in the survival time compared with the control group. It was also observed that the survival time in group 1 was significantly higher than in groups 2 (χ2 = 5.55, P = 0.018) and 5 (χ2 = 5.55, P = 0.018). Still, there was no significant difference between groups 2 and 5 in terms of survival time (χ2 = 0.55, P = 0.56) (Figure 5). The chi-square test results, which were used to check the Metastasis Index in the different studied groups, showed that AMP treatment on days 10 and 20 significantly reduces the amount of tumor cell metastasis. While LND on days 10 and 20 does not affect the metastasis of these cancer cells. In addition, the simultaneous treatment of leg AMP and LND on the 20th day after injection significantly reduces the metastasis rate of tumor cells (Table 1). Furthermore, the analysis tests showed that there are no significant differences in the metastasis rate of breast cancer cells between groups 1 and 2 (χ2 = 1, P = 0.32), 1 and 5 (χ2 = 2.17, P = 0.14) and 2 and 5 (χ2 = 0.39, P = 0.53). A summary of the content is given in Table 2.
Kaplan-Meier curves in Balb/C mice injected with 4T1 breast cancer cells subjected to early amputation (AMP) (group 1), delay AMP (group 2), early lymph node dissection (LND) (group 3), delay LND (group 4), AMP + LND (group 5), only cancer cells injection (group 6), and no intervention (group 7)
| Groupings | Metastasis Index | χ2 | P-Value | |
|---|---|---|---|---|
| No | Yes | |||
| Early AMP (G1) | 7 (100) | 0 | 11.67 | 0.001 |
| Delay AMP (G2) | 6 (85.7) | 1 (14.3) | 8.19 | 0.004 |
| Early LND (G3) | 1 (16.7) | 5 (83.3) | 0 | 1 |
| Delay LND (G4) | 2 (28.6) | 5 (71.4) | 0.36 | 0.55 |
| AMP + LND (G5) | 5 (71.4) | 2 (28.6) | 5.4 | 0.02 |
| Disease control (G6) | 2 (16.7) | 10 (83.3) | - | - |
| Healthy control (G7) | 7 (100) | 0 | 9.52 | 0.02 |
Abbreviations: AMP, amputation; LND, lymph node dissection.
a Values are expressed as No. (%).
| Clinical Translation | Number of Mice Survived for 120 Days | LN Status | Site of Metastasis | Metastasis/Numbers | Surgical Intervention | Tumor Cell Injection | Group Number | |
|---|---|---|---|---|---|---|---|---|
| 20 Days (Late) | 10 Days (Early) | |||||||
| Early primary tumor management | 6 | Normal LNs | - | None | - | AMP | + | Group 1, 7 mice |
| Late primary tumor management | 1 | Normal LNs | Lung | 1 of 7 mice | AMP | - | + | Group 2, 7 mice |
| Removal of normal regional LNs (early) | 1 | No LN metastases | Lung/liver | 5 of 6 mice | - | LND | + | Group 3, 7 mice |
| Late removal of normal regional LNs | 0 | No LN metastases | Lung/liver | 5 of 7 mice | LND | - | + | Group 4, 7 mice |
| Conventional surgical management | 0 | No LN metastases | Lung/liver | 2 of 7 mice | AMP + LND | - | + | Group 5, 7 mice |
| No intervention | 0 | Normal LNs | Lung/liver | 10 of 12 mice | - | - | + Diseased group | Group 6, 12 mice |
| Healthy cases | 7 | Normal LNs | None | None | - | - | No injection (healthy group) | Group 7, 7 mice |
Abbreviations: AMP, amputation; LND, lymph node dissection.
5. Discussion
According to the results of this work, primary management is essential in controlling or treating tumors. When we eliminate the tumor, we have the best OS compared to when only LND is performed. This finding aligns with previous studies suggesting that LND alone does not confer survival benefits in solid tumors, including breast cancer. Even early local management has a better OS than delaying local management. One possible explanation is that metastatic spread may occur before lymph node involvement, questioning the necessity of prophylactic lymphadenectomy. Also, LND without removing the primary tumor is futile work. When LND and primary control are done together (like what is generally done in patients with cancer), and the lymph nodes are pathologically negative, it doesn't have any superiority compared to only removing the primary tumor. Indeed, in our theory, lymph nodes are housekeepers, and we shouldn't remove them until tumors involve them. This aligns with human studies showing no survival advantage of CLND in early-stage breast cancer. The findings could support a shift toward more conservative surgical approaches, limiting unnecessary lymphadenectomy in breast cancer treatment guidelines. On the other hand, it's better not to remove healthy housekeepers because they are the mainstay of resistance against tumor cells. It should be emphasized that all lymph nodes involved by the tumor (positive lymph node) should be removed. In this study, because all lymph nodes were negative, no difference in OS was observed between group 2 (delay AMP) and 5 (AMP + LND). Looking at the different groups' results, we hypothesized two theories: (1) Local management of cancers is the main effective surgery, especially very soon after diagnosis (group 1, 2, and 5); (2) breast cancer is a systematic disease; metastasis may happen without regional lymph node involvement (group 4 and 5).
Lymph node invasion is the first sign of tumor progression in most epithelial malignancies. Compared to CLND, many studies have shown that limited LND has no difference in OS. Javadi et al. showed that limited axillary LND (ALND) (4 - 6 nodes) in breast cancer has no inferiority to more LND (10 nodes) in cases where we don't have palpable lymph nodes, and we can't do SLN biopsy (SLNB) (19). In another study, Negahi et al. showed that in early gastric cancer, the limitation of LND can improve OS (20). Giuliano et al. demonstrated that SLNB alone, compared with ALND, does not have inferior survival in patients with limited SLN metastatic breast cancer (21). Also, Krag et al. noted when the SLN is negative, SLNB with no ALND is a safe, appropriate, and effective therapy for breast cancer patients (22). This shows that at least excessive LND doesn't have any superiority to limited LND dissection. Also, Zanghi et al. showed that even if axillary micrometastasis is detected in SLN in breast cancers, ALND could be avoided (23). Excessive negative lymphadenectomy has many complications. Lymphedema is the most common complication of ALND in breast cancer (24). In a retrospective study, Quan et al. showed that the outcome of a more negative lymph node retrieval from colon cancer is better in pN1 compared to pN2 patients. The immune system is strong, and the tumor couldn't overcome the lymph nodes in pN1 patients, but in pN2 patients, cancer overcomes the immune system (25). In our theory, the immune system becomes weaker when more negative lymph nodes are dissected in the same pathology N stage. In patients with breast cancer, when SLN is involved microscopically, more extensive lymph node removal is not indicated (26, 27). In breast cancer, melanoma, and some other cancers, recent data promote surgeons to remove SLN and leave other not-involved LNs. As far as the scientific data suggest, during tumor cell metastasis, the structure and function of SLN, including hyperplasia and lymphangiogenesis, undergo some changes (28). In early-stage colon cancer, lymphogenic and hematogenic tumor cell dissemination have equal importance, questioning the traditional idea of sequential metastatic spread (29). In breast cancer, the SLN infiltration does not predict the occurrence of bone marrow micrometastasis (BMM), indicating that the thought of sequential spread must be seriously doubted. However, if indicated, SLN mapping and finding out small tumor node infiltration and BMM are better tools for prognostic factors in solid tumors (26, 29).
5.1. Conclusions
The most effective treatment for solid tumors is local management and cancer removal. The next step is to assess the spread of the tumor by evaluating the regional lymph nodes. If tumors involve the lymph nodes, they should be removed, but removing negative lymph nodes will not affect OS and may be unsuitable. Our findings suggest that lymphadenectomy without primary tumor removal does not improve survival and may not be necessary in cases where lymph nodes remain uninvolved. Breast cancer is a systematic disease; metastasis may happen without regional lymph node involvement. More studies are needed to know whether the lymph nodes are involved and whether to remove them or not. Future research should explore whether selective lymph node biopsy can replace extensive lymphadenectomy in solid tumors and assess the immune response role of uninvolved lymph nodes. Additionally, studying lymphatic drainage pathways beyond inguinal lymph nodes may help clarify their role in metastatic progression.
5.2. Limitations
The limitation of this study was that we could not evaluate organs other than the liver and lung for metastasis. In mice, approximately half of the hind leg drainage enters the inguinal LNs and the other half drainage to iliac nodes (30). This could be why we didn't find a positive lymph node. Also, if we had done the intervention later than 20 days, finding a positive lymph node would be possible.