1. Background
Colorectal cancer (CRC), which includes both colon and rectal cancers, presents a significant public health challenge due to its increasing incidence and mortality rates. In 2020, it was the third most common cancer worldwide, with 1 931 590 new cases (10% of all cancers) and 935 173 deaths attributed to the disease (1, 2). In Iran, CRC ranks as the second most prevalent cancer among men and the third among women, with an incidence rate of approximately 10 to 12 cases per 100 000 people, resulting in thousands of new diagnoses each year (3).
Early diagnosis and tumor staging are essential for the survival of patients with CRC, as timely detection enhances treatment outcomes. Survival rates are influenced by factors such as age, sex, and tumor stage, with the stage and location of the tumor being critical in determining the risk of metastasis and recurrence (4, 5). Recent studies indicate a rising incidence of CRC among individuals under 50 (6). This rise is associated with factors such as smoking, obesity, dietary habits, and genetic predispositions (7). Furthermore, the accumulation of mutations in key genes, including tumor suppressor genes and oncogenes, plays a significant role in the development of CRC (8).
Human leukocyte antigens (HLAs) class I expression has emerged as a significant factor in tumor progression and metastasis, with decreased levels aiding tumor cells in evading immune detection and promoting their spread HLA class I downregulation correlates with adverse clinical outcomes and varies in tumor cells due to genetic instability, impacting patient survival. Studies have revealed that lower HLA class I expression is often linked to poor survival rates in colon cancer patients (9, 10) and is associated with worse prognoses across other cancer types (11-13). This downregulation in primary tumors and metastatic lymph nodes suggests that reducing HLA class I molecules allows tumor cells to escape immune surveillance, potentially leading to poorer outcomes (14, 15).
Recent advancements in cancer treatment have increased the emphasis on survival analysis and cure modeling. Traditional survival analysis methods, such as Kaplan–Meier estimators and log-rank tests, have limitations in addressing patient subpopulations that can be cured (16). Cure models effectively differentiate between patients likely to achieve long-term survival and those at risk of succumbing to the disease. These models categorize the analyzed population into susceptible and non-susceptible groups, often visualized through a Kaplan–Meier survival function plot exhibiting a prolonged stable plateau with significant censoring (17, 18).
2. Objectives
This study uses a parametric defective Marshall-Olkin Weibull model to estimate the cure fraction in CRC data. By integrating changes in HLA class I expression levels and metastatic status into our analysis, this investigation seeks to enhance our understanding of the interactions between genetic factors, HLA class I expression changes, and patient outcomes such as death in CRC.
3. Methods
This study evaluates data from a historical cohort of 226 patients diagnosed with primary CRC between February 2005 and October 2015 at the Cancer Prevention Division of the Research Center for Gastrointestinal and Liver Diseases (RCGLD) at Shahid Beheshti University of Medical Sciences in Tehran, Iran. Eligibility criteria included adults aged 18 years and older with a confirmed CRC diagnosis. Participants’ comprehensive medical records were meticulously reviewed to confirm inclusion criteria.
We assessed independent variables influencing overall survival, including age, sex, tumor location (colon vs. rectum), TNM stage, grade of differentiation (well/moderate vs. poor), age at diagnosis, family history, microsatellite instability (MSI), CD8 and Ras mutation status, and HLA expression class I, as detailed in references (19-21).
The Kaplan–Meier method was employed to estimate the time to cure, with the cure fraction calculated as the difference between the survival curve and zero survival probability. We utilized the defective Marshall-Olkin extended Weibull model to investigate the relationships between these variables and survival time. The survival function is expressed as:
Where γ > 0, β represents the coefficient vector, and x denotes the covariate vector. The cure rate for t > 0 is linked to the covariate by the setting r(x) = -exp(β′x). The cure rate of S(t) can be computed as follows (17):
Where β′x increases, the cure rate trends toward 1; conversely, if β′x decreases, the cure rate trends toward 0.
Parameter estimation was conducted utilizing the maximum likelihood method, with model selection focused on achieving the lowest Akaike information criterion (AIC). Statistical analyses were performed using R software (version 4.2.1; R Foundation for Statistical Computing; www.r-project.org), with significance set at P < 0.05. The multivariate defective Marshall-Olkin Weibull model was used to estimate the cure rate, quantifying the odds of cure via odds ratio (OR) and the risk of death through hazard ratio (HR). This model assumes that the cumulative hazard is bounded from above in the presence of a cure fraction, thereby allowing for biological interpretation in the context of cancer. Variable inclusion was determined by minimizing AIC, and data visualization was excluded using the ggplot2 package.
4. Results
The patient follow-up procedure was completed in March 2015, with a mean follow-up time of 3.93 years (median: 3.1 years; range: 0.1 - 10.81 years, SD: 2.68 years). Among the 226 participants, 122 (54%) were men and 104 (46%) were women, with an average age of 57.15 ± 11.15 years (range: 28 to 88 years). Analysis of HLA gene expression revealed that 35.4% (n = 80) of participants showed loss of expression, 53.1% (n = 120) exhibited downregulation, and 11.5% (n = 26) displayed high expression. Regarding metastasis status, it was demonstrated that 81.4% (n = 184) of participants had metastasis, while 18.6% (n = 42) did not (Table 1).
| Variables | Values |
|---|---|
| Sex | |
| Female | 104 (46) |
| Male | 122 (54) |
| Location of the tumor | |
| Left colon | 140 (61.9) |
| Right colon | 86 (38.1) |
| Differentiation | |
| Poor | 164 (72.6) |
| Moderate well | 62 (27.4) |
| Mucinous characteristics | |
| No | 196 (86.7) |
| Yes | 30 (13.3) |
| TNM stage | |
| Stage I | 23 (10.2) |
| Stage II | 107 (47.3) |
| Stage III | 76 (33.6) |
| Stage IV | 20 (8.8) |
| Vital status | |
| Dead | 47 (20.8) |
| Alive | 179 (79.2) |
| CD8 | |
| Weak | 121 (53.5) |
| Strong | 105 (46.5) |
| Ras mutation | |
| Mutant | 27 (11.9) |
| Wild | 199 (88.1) |
| Chemotherapy | |
| No | 131 (58.0) |
| Yes | 95 (42.0) |
| Anemia | |
| No | 192 (85.0) |
| Yes | 34 (15.0) |
| IBD | |
| No | 180 (79.6) |
| Yes | 46 (20.4) |
| Family history | |
| No | 164 (72.6) |
| Yes | 62 (27.4) |
| Diabetes | |
| No | 45 (80.1) |
| Yes | 181 (19.9) |
| Metastasis | |
| No | 184 (81.4) |
| Yes | 42 (18.6) |
| MSI status | |
| Slow | 196 (86.7) |
| High | 30 (13.3) |
| HLA class I | |
| Down | 120 (53.1) |
| Loss | 35.4 (35.4) |
| High | 26 (11.5) |
Abbreviations: MSI, microsatellite instability; HLA, human leukocyte antigen.
a Values are expressed as No. (%).
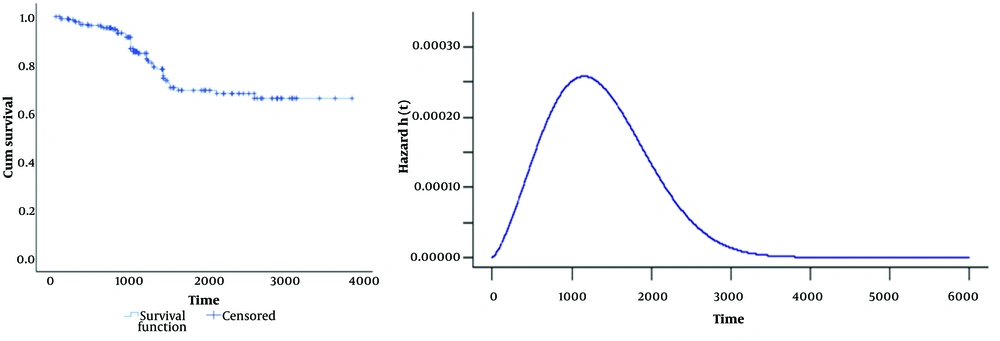
The Kaplan–Meier plot in Figure 1 (left) demonstrates remarkable long-term survival among colon cancer patients, with the survival rate remaining above zero and stabilizing at 2 658 days. This extended survival period points to a considerable cure rate, estimated at 66% (95% CI: 60% - 70%), validating the use of cure models to analyze these patient outcomes. Figure 1 (right) displays the hazard function of the defective Marshall-Olkin Weibull model, further characterizing the survival distribution.
Using a multivariate defective Marshall-Olkin Weibull model, we estimated the cure rate for colon cancer, selecting variables to minimize AIC. The primary significant variable was HLA downregulation, with a P-value of 0.0001 and a coefficient (β) of -1.53, indicating a strong negative relationship. Patients without downregulated HLA (high or lost HLA) had a cure rate 4.75 times higher than those with downregulated HLA (Table 2). Metastasis exhibited a P-value of 0.001 and a coefficient (β) of -1.26, demonstrating a significant negative association. Patients without metastasis had a cure rate 3.63 times greater than those with metastasis, emphasizing the critical roles of HLA downregulation and metastasis in the model (Table 2).
| Variables | β | P-Value | Odds Cure (CI) |
|---|---|---|---|
| Intercept | 1.8 | ||
| Downregulation of HLA | -1.53 (-2.32, -0.74) | 0.0001 | 0.22 (0.1, 0.47) |
| Metastasis | -1.26 (-2.06, -0.52) | 0.001 | 0.28 (0.13, 0.6) |
Abbreviation: HLA, human leukocyte antigen.
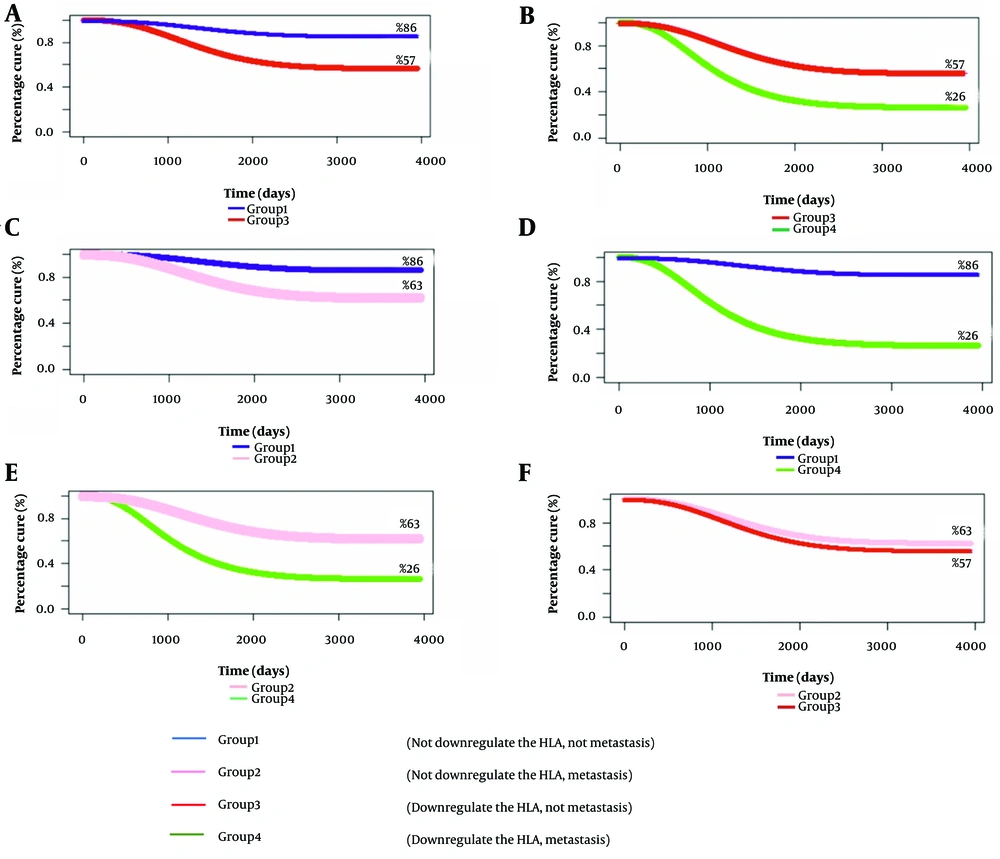
The relationship between HLA expression and metastasis provides essential insights (Figure 2). Patients with low HLA expression have a cure rate of 62%, similar to the 57% cure rate in patients with metastasis without low HLA expression (Figure 2F). Conversely, non-metastatic individuals with low HLA expression have a cure rate of 57%, significantly lower than the 86% cure rate of those with high or absent HLA expression (Figure 2A). In patients with HLA Class I expression and metastasis, the cure rate decreases sharply from 57% to 26% (Figure 2B). These findings highlight the impact of changes in HLA expression and metastatic progression on patient outcomes, suggesting further investigation into its therapeutic implications (Table 3).
| Variables | Cure Rate Calculated by the Defective Model | Survival Rate Estimate by Kaplan-Meier | Total CRC Cases | Number Died of CRC |
|---|---|---|---|---|
| (Not downregulate the HLA, not metastasis) or group 1 | 0.86 | 0.89 | 92 | 7 |
| (Not downregulate the HLA, metastasis) or group 2 | 0.62 | 0.56 | 14 | 3 |
| (Downregulate the HLA, not metastasis) or group 3 | 0.57 | 0.55 | 92 | 24 |
| (Downregulate the HLA, metastasis) or group 4 | 0.26 | 0.28 | 28 | 13 |
Abbreviations: CRC, colorectal cancer; HLA, human leukocyte antigen.
Table 3 reveals that among 92 patients with high HLA expression and no metastasis, 7 died, compared to 24 out of 92 patients with low HLA expression. Among the 14 patients with metastasis and high HLA levels, 3 died, while 13 out of 28 patients with reduced HLA expression also experienced mortality. The defective Marshall-Olkin curve estimates cure rates for various scenarios, displayed alongside cure values in Figure 2 for enhanced clarity.
5. Discussion
This study examines the modeling of survival data that involves death events and a cure fraction. Cure models are applicable when some individuals are immune to the event, dividing them into 2 categories: the susceptible group, which experiences short-term survival, and the non-susceptible group, which enjoys long-term survival and is considered immune. Notably, these models revert to standard survival models when the cure fraction is absent.
The defective Marshall-Olkin Weibull model has provided insights into the long-term survival dynamics of colon cancer patients. Previous studies have shown that this model is more suitable than parametric models for estimating cure rates because it does not require the introduction of an additional parameter to the survival model for estimating cure rates estimate (17). The application of this model in melanoma disease was also shown in another study (22).
Research has shown that patients with high/low HLA expression have a cure rate approximately 4.75 times higher than those with downregulated HLA. Additionally, the odds of a cure are 3.63 times greater for patients without metastasis. These findings are consistent with existing literature that emphasizes the role of HLA class I in tumor immune evasion and its impact on cancer progression and treatment outcomes (23, 24).
The statistical analysis performed using the defective Marshall-Olkin Weibull model shows significant negative correlations with HLA downregulation (P = 0.0001, β = -1.53) and metastasis (P = 0.001, β = -1.26). These results indicate that HLA downregulation enhances a tumor's ability to evade the immune system, which is associated with decreased survival outcomes (24).
This study emphasizes the essential role of changes in HLA class I expression in predicting outcomes for colon cancer, particularly concerning downregulation and metastasis. Patients with high or lost HLA expression had a cure rate of 86%, whereas those with downregulated HLA had a cure rate of 57%, and patients with metastasis had a cure rate of only 26%. These findings indicate that the downregulation of HLA class I significantly impacts survival and cure rates. That HLA class I downregulation significantly affects survival and the cure rate (10, 15, 25).
Interestingly, cure rates among patients with low HLA expression but no metastasis (57%) are similar to those with high/loss HLA expression but with metastasis (62%). This indicates that HLA downregulation may aid metastatic progression, impacting survival. The long-term follow-up data, averaging 3.93 years, shows a plateau in the survival curve, suggesting that some patients may achieve long-term survival. With an estimated cure rate of 66%, some patients may experience lasting responses to treatment despite challenges from HLA downregulation and metastasis (25).
The similarities in cure rates between individuals with low HLA expression and those with metastasis indicate that low HLA expression may weaken the immune response, making patients more prone to metastasis or reducing their treatment success. This highlights the need for further research into HLA expression as a potential marker for prognosis. Understanding how HLA influences tumor progression and treatment outcomes could lead to targeted strategies to boost immune responses in patients with low HLA expression (26-28).
Nevertheless, the generalizability of our findings may be limited by the specific demographics of our cohort, which primarily consisted of participants aged 28 to 88 years, with a mean age of 57.15 years and a 10-year follow-up period of 2005 to 2015. To strengthen the applicability of these results, future research should encompass larger, multi-center studies involving diverse populations, including younger individuals in more recent cohort studies with longer follow-up and those from varied ethnic backgrounds. Such efforts will deepen our understanding of the role of HLA expression in metastasis and its implications for patient outcomes across broader settings.
5.1. Clinical Interpretation
This study underscores the critical role of HLA class I expression in guiding treatment strategies for CRC. The studies collectively highlight that downregulation or low HLA Class I expression in CRC is associated with immune evasion and may contribute to metastatic progression. Incorporating HLA assessment into routine clinical practice may enable more personalized treatment plans, enhancing patient outcomes (9, 10).
The low HLA Class I molecules impair the presentation of tumor antigens to cytotoxic T lymphocytes, allowing tumor cells to escape immune surveillance. This immune escape mechanism is linked to poorer clinical outcomes and may influence the effectiveness of immunotherapies (23, 24). By tailoring treatment strategies based on HLA status, clinicians could significantly improve survival rates and overall patient quality of life.