1. Background
Metastatic gastrointestinal cancers, which include malignancies such as gastric, colorectal, and pancreatic cancers, are among the most aggressive and treatment-resistant types of cancer globally. These malignancies account for a substantial proportion of cancer-related deaths and exhibit poor outcomes, particularly in metastatic stages (1). For example, gastric adenocarcinoma with a five-year survival rate of less than 20% in metastatic cases is the most common malignancy of the gastrointestinal tract in Iran. Despite significant advancements in chemotherapeutic regimens, including FOLFOX (oxaliplatin, leucovorin, and 5-fluorouracil) and FOLFIRI (irinotecan-based regimens), long-term outcomes remain unsatisfactory (2). Moreover, systemic chemotherapy often leads to debilitating side effects, such as severe hematological toxicity, neuropathy, and gastrointestinal symptoms, which further limit its utility. This therapeutic gap underscores the urgent need for innovative strategies to enhance efficacy while minimizing adverse effects (3).
Hyperthermia, defined as the controlled elevation of tissue or whole-body temperature (39°C - 42°C), has emerged as a promising adjunctive therapy for cancer. Historically, the therapeutic effects of hyperthermia were recognized as early as 3000 BCE (4). In modern oncology, hyperthermia is primarily used to enhance the efficacy of chemotherapy and radiotherapy. It exerts a range of biological effects, including direct tumor cytotoxicity, improved tumor perfusion, increased drug delivery, and the activation of immune responses (5). Hyperthermia can be administered regionally or systemically. Regional hyperthermia targets specific tumor sites, while systemic hyperthermia elevates the entire body’s core temperature, making it suitable for metastatic and disseminated malignancies. The primary mechanism involves disrupting the tumor microenvironment, where elevated temperatures increase blood flow and oxygenation to hypoxic tumor regions, rendering them more susceptible to cytotoxic agents (6).
Several recent studies have demonstrated the potential of hyperthermia as an adjunct to systemic therapies. Deniz et al. (7) investigated radiofrequency hyperthermia (oncothermia) combined with chemotherapy in metastatic colorectal cancer. This study, involving 25 patients, reported an impressive objective response rate of 96%, with no grade III–IV toxicities. The findings highlighted hyperthermia's role in overcoming chemoresistance. Zwischenberger et al. (8) introduced hyperthermic extracorporeal applied tumor therapy (HEATT®) for advanced, chemo resistant malignancies. This novel approach involves homogeneous heating via veno-venous perfusion and has shown promise in patients unresponsive to conventional chemotherapy. Issels et al. (9) reported systemic antitumor effects in metastatic rhabdomyosarcoma using regional hyperthermia combined with low-dose chemotherapy. This approach activated natural killer (NK) cells and cytotoxic T-cells, inducing regression of distant metastases. Mulens-Arias et al. (10) developed a precision approach using gold nanoparticles activated by near-infrared light to induce tumor-selective hyperthermia. This strategy demonstrated reduced systemic toxicity and enhanced tumor necrosis in preclinical models.
Hyperthermia enhances tumor sensitivity through several mechanisms. Elevated temperatures increase the permeability of tumor vasculature, facilitating deeper penetration of chemotherapeutic agents. Additionally, hyperthermia destabilizes cellular membranes and proteins, leading to apoptosis and necrosis in malignant cells. By inducing heat shock proteins (HSPs), hyperthermia improves antigen presentation, activates dendritic cells (DCs), and stimulates cytotoxic T-cells (11). Toraya-Brown and Fiering (12) demonstrated that hyperthermia can elicit systemic antitumor immunity, further enhancing its therapeutic potential. Furthermore, hyperthermia disrupts repair mechanisms in DNA-damaged cells, counteracting chemoresistance.
Despite these promising findings, the adoption of hyperthermia in routine oncology remains limited by logistical and technical challenges. The variability in outcomes across studies further highlights the need for standardized protocols and comprehensive clinical trials. Monitoring adverse effects of combined chemotherapy and hyperthermia protocols is crucial for evaluating the safety profile of the treatment. The combined treatment protocol can lead to various hematologic and non-hematologic adverse effects. These include severe hematologic toxicities, such as leukopenia and anemia, as well as non-hematologic effects like diarrhea, neuropathy, stomatitis, nausea, and vomiting, which significantly impact quality of life of patients and treatment adherence (13).
2. Objectives
This study aims to assess the efficacy and safety of combining systemic hyperthermia with chemotherapy in patients with metastatic gastrointestinal cancers. The specific objectives include assessing the impact on response rates, evaluating the toxicity profiles and tolerability of combined therapy. By addressing these gaps, this research seeks to generate evidence supporting the integration of hyperthermia as a standard adjunctive treatment in metastatic cancer care.
3. Methods
3.1. Study Design
This pilot study was conducted to evaluate the effects and adverse outcomes of systemic chemotherapy combined with Whole-body hyperthermia (WBH) in patients with metastatic gastrointestinal cancers. The study was performed at Shohada Tajrish Hospital, Tehran, Iran between September 2015 and January 2016.
3.2. Patient Selection
Patients were eligible if they had an eastern cooperative oncology group (ECOG) performance status (PS) of ≤ 2, were over 18 years of age, had a life expectancy of at least 3 months, did not have severe medical comorbidities or other malignancies, and had a creatinine clearance of ≥ 60 mL/min. Adequate hepatic function was defined as total bilirubin levels of ≤ 1.5 mg/dL, alkaline phosphatase and aspartate aminotransferase levels of ≤ 3 times the normal range. Sufficient bone marrow function required a white blood cell count (WBC) of ≥ 3 × 109/L cells, an absolute granulocyte count of ≥ 1.5 × 109/L cells, and a platelet count of ≥ 100 × 109/L. Additionally, normal serum electrolyte values were necessary.
Patients were excluded from the study if they had coronary artery disease, angina pectoris, congestive heart failure, serious arrhythmias or severely compromised respiratory status.
3.3. Ethical Considerations
This study received approval from the Medical Ethics Committee (approval No. IR.SBMU.SM.REC.1394.40) and was conducted in accordance with the Declaration of Helsinki's ethical principles, ensuring respect for participants, informed consent, and the protection of vulnerable populations. All participants provided written informed consent prior to enrollment.
3.4. Treatment
Hyperthermia was carried out on day 1 of each chemotherapy cycle using HECKEL HT-3000 (Heckel medizintechnik GmbH, Esslingen, Germany) device.
The HECKEL HT-3000 employs water-filtered infrared radiation (wIRA) delivered through four emitters targeting the chest, alongside two heating elements to warm the air within its tent-like structure. Initially, subjects were positioned on a bed equipped with the warming device, where their heart rate, electrocardiogram, oxygen saturation, mean arterial pressure, and core temperature (measured via a rectal probe) were continuously monitored. Patients received alprazolam for sedation. During the WBH procedure, a core temperature of 38.5°C to 40°C was achieved, and patients were maintained at this temperature plateau for a duration of 1.5 hours and received chemotherapy concurrently. Chemotherapy drugs were administrated at 80% of standard dose. After the end of WBH, patients were monitored for 24h in hospital for safety.
3.5. Clinical Response and Toxicity Evaluation
Patients were assessed at each course of chemotherapy for side effects, including hematologic and biochemical parameters. Toxicities were assessed using the National Cancer Institute (NCI) common toxicity criteria. Responses were evaluated based on the following definitions: A complete remission (CR) indicated the total disappearance of all measurable and assessable tumor disease, along with the normalization of tumor markers and laboratory values. A partial response (PR) was designed as a reduction of ≥ 50% in the sum of the products of the perpendicular diameters of all measured lesions, with no increase in the size of any lesion and no appearance of new lesions. Stable disease (SD) was defined as a steady state or a response less than a PR, but with no disease progression for at least four weeks.
4. Results
This research was conducted from September 2015 to January 2016.The location of the primary tumor, the sites of metastases, the number of previous chemotherapies, and the patients' PS before starting treatment are shown in Table 1.
| Demographic Information | Patients; No. (%) |
|---|---|
| Total patients | 20 (100) |
| Age | |
| Mean age | 54.8 |
| Age range (y) | 30 - 75 |
| Gender | |
| Female | 10 (50) |
| Male | 10 (50) |
| Age group | |
| ≤ 30 | 0 |
| 31 - 40 | 1 |
| 41 - 50 | 2 |
| 51 - 60 | 3 |
| 61 - 70 | 4 |
| ≥ 71 | 10 |
| Primary tumor site | |
| Colon | 10 (50) |
| Rectum | 3 (15) |
| Stomach | 4 (20) |
| Pancreas | 2 (10) |
| Gallbladder | 1 (5) |
| Metastatic sites | |
| Liver | 14 (50) |
| Lung | 5 (17.85) |
| Omentum | 5 (17.85) |
| Lymph nodes | 4 (14.3) |
| Previous chemotherapy lines | |
| 0 | 7 (35) |
| 1 | 5 (25) |
| 2 | 4 (20) |
| 3 | 4 (20) |
| PS | |
| 0 | 6 (30) |
| 1 | 7 (35) |
| 2 | 7 (35) |
Abbreviation: PS, performance status.
Understanding the baseline demographic and clinical characteristics of the study population is
essential for interpreting the effectiveness and safety outcomes of any therapeutic intervention.
According to Table 1, the study involved 20 patients with a balanced gender distribution, comprising 50% males and 50% females, and an average age of 54.8 years, with ages ranging from 30 to 75 years. Most patients were aged between 51 and 60 years. Primary tumor locations varied, with the colon being the most common site (50%), followed by the stomach (20%), rectum (15%), pancreas (10%), and gallbladder (5%). Most patients (35%) had not undergone previous chemotherapy sessions, while the remaining had varying numbers of prior cycles. Performance status was distributed relatively evenly, with 30% having PS 0 and the remaining equally split between PS 1 and PS 2. These characteristics indicate a relatively well-functioning cohort undergoing treatment.
The combined chemotherapy and hyperthermia treatment protocol can lead to various hematologic and non-hematologic adverse effects. Monitoring and documenting these adverse effects are crucial for evaluating the safety profile of the treatment. The results section categorizes adverse effects by severity grades based on the National Cancer Institute's toxicity criteria and explores gender-based differences in toxicity. This analysis provides a comprehensive understanding of the tolerability of the treatment regimen and highlights potential areas for supportive care interventions.
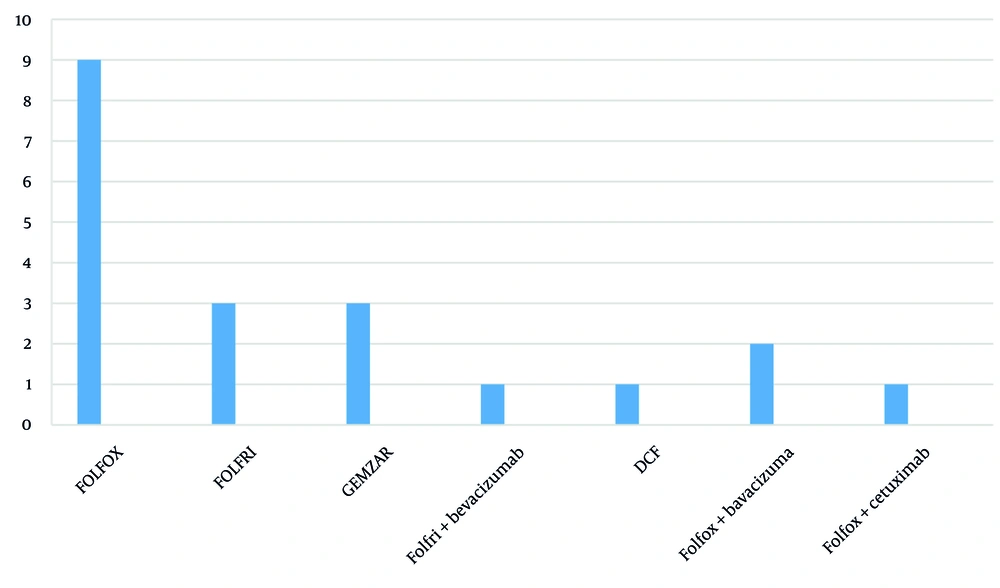
All tumors were of the adenocarcinoma type, and all patients tolerated hyperthermia, and a total of 101 chemotherapy sessions were conducted, of which 91 sessions were combined with hyperthermia. The average number of chemotherapy cycles was 4.9, and the average number of hyperthermia sessions was 4.6. The most commonly used chemotherapy protocol was FOLFOX, without target agent (Figure 1).
Table 2 outlines the adverse effects observed during combined chemotherapy and hyperthermia treatment, categorized by severity grades based on the National Cancer Institute's toxicity criteria.
| Treatment Adverse Effects | Grade 4 | Grade 3 | Grade 2 | Grade 1 | Grade 0 |
|---|---|---|---|---|---|
| Leukopenia | 2 (2) | 5 (5) | 14 (15) | 33 (36) | 37 (41) |
| Anemia | 0 (0) | 2 (2) | 22 (24) | 39 (43 ) | 28 (30) |
| Thrombocytopenia | 0 (0) | 0 (0) | 3 (3) | 4 (4) | 84 (92) |
| Diarrhea | 0 (0) | 3 (3) | 4 (4) | 41 (45) | 43 (47 ) |
| Nausea | 0 (0) | 3 (3) | 19(21) | 34 (37) | 35 (38 ) |
| Vomiting | 0 (0) | 2 (2) | 10 (11) | 39 (43) | 40 (44) |
| Neuropathy | 0 (0) | 4 (4) | 18 (20) | 39 (43) | 30 (33) |
| Stomatitis | 0 (0) | 3 (3) | 10 (10) | 30 (32) | 48 (54) |
a Values are expressed as No. (%).
The most frequently observed grade 1 adverse effects were diarrhea (45%), and vomiting (43%), indicating that gastrointestinal symptoms were common. Grade 2 effects were primarily anemia (24 %) and neuropathy (20%), highlighting hematologic and nervous system impacts. Grade 3 effects were dominated by leukopenia (5%) and neuropathy (4%), while only leukopenia was reported as a grade 4 adverse effect (2%). This distribution indicates that leukopenia requires close monitoring as a potentially severe adverse effect of the treatment protocol
Evaluating treatment response rates is a critical component of any clinical study assessing therapeutic effectiveness. In this study, the clinical responses are categorized into CR, PR, SD, and PD. These categories provide a framework for assessing the impact of combined therapy on tumor control and disease progression.
The majority of patients (40%) exhibited PRs to the treatment, indicating that the combined protocol was effective in reducing tumor burden in a significant portion of the cohort. Complete responses were rare (5%) and only patients who achieved CR had rectal cancer with metastasis to liver and omentum , while stable disease was observed in 35% of patients. Disease progression occurred in 20% of patients, underscoring the need for more effective therapeutic combinations to achieve better control of metastatic disease.
In patients who responded to treatment (CR, PR, SD), the maximum time to the onset of clinical symptoms indicating disease progression (TIME TO PROGRESSION) was around 10 months, and the mean TTP (time to progression) was approximately 4.5 months.
All patients who responded to treatment (CR, PR) underwent 6 sessions of hyperthermia.
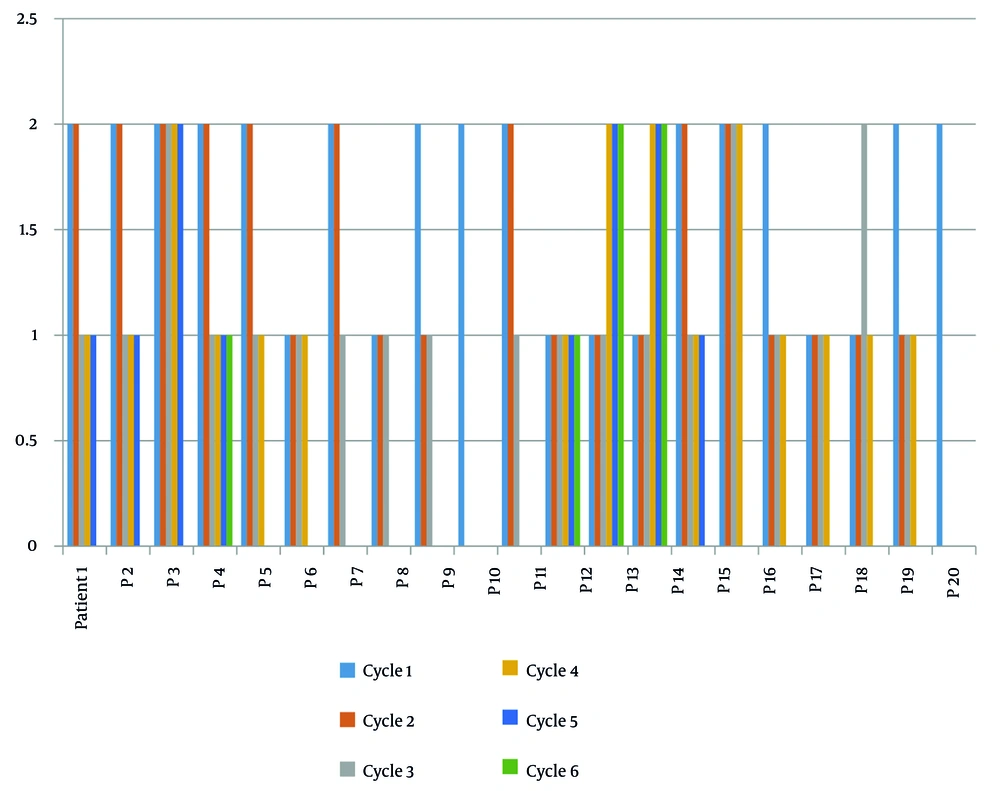
After excluding four patients who were unable to continue treatment, the clinical status of patients (PS) was evaluated after each chemotherapy cycle. It was found that the PS either remained stable or showed an improving trend (Figure 2).
The figure demonstrates that most patients experienced either stable or improving performance status scores throughout the treatment course. The positive trend in PS scores suggests that combined chemotherapy and hyperthermia treatment helped maintain or enhance patients' functional capacity, contributing to better overall quality of life during the treatment period.
5. Discussion
This pilot study aimed to evaluate the feasibility, tolerability, and potential therapeutic effects of combining WBH with chemotherapy in patients with metastatic gastrointestinal cancers. Hyperthermia is known to modulate tumor biology in several ways:
Vascular effects: Hyperthermia can increase blood flow and oxygenation within tumors by causing local vascular dilation, which may improve drug delivery and enhance the effects of chemotherapy
Cell membrane permeability: Elevated temperatures increase cell membrane fluidity, potentially enhancing cellular uptake of chemotherapeutic agents
Immune system stimulation: Hyperthermia can stimulate immune responses by inducing HSPs, activating DCs, and promoting T-cell responses against tumor cells. This immunological effect could contribute to improve clinical outcomes.
Our findings demonstrated that WBH without general anesthesia is feasible and generally well-tolerated. The hyperthermia protocol involved heating patients to a core temperature of 39 - 40°C over 1.5 hours, maintaining this temperature for 1 hour, followed by a 1-hour cooling phase. Notably, patients were monitored closely using rectal probes to measure core body temperature, and a saline infusion was administered to prevent dehydration. These measures ensured that patients could safely undergo the hyperthermia sessions.
The results align with previous studies, such as those by Hegewisch-Becker et al. and Koga et al., which reported the efficiency of WBH combined with chemotherapy (14, 15). However, a key difference in our study is that our patients were awake and able to report discomfort, negating the need for general anesthesia or invasive temperature monitoring probes, such as esophageal probes. This highlights a critical distinction in the application of WBH: Patients can tolerate hyperthermia without general anesthesia, thereby reducing associated risks and recovery times.
Our findings indicated that hyperthermia did not exacerbate chemotherapy-related toxicities significantly. The observed grade 4 adverse event rate was low, with only two patient experiencing severe leukopenia (16). Similarly, grade 3 toxicities were primarily limited to leukopenia and neuropathy, occurring in less than 5% of cases (17, 18). These outcomes suggest that the combination of WBH and chemotherapy is a viable option for enhancing therapeutic effects without imposing excessive toxicity.
One significant observation in our study was the impact of WBH on cardiovascular parameters. Consistent with the literature, we observed an increase in heart rate and cardiac output during hyperthermia sessions. One patient experienced ischemic heart symptoms, underscoring the necessity of continuous cardiac monitoring during WBH procedures. This finding is in line with studies conducted by Hegewisch-Becker et al. and Koga et al., which emphasized the importance of monitoring to mitigate potential cardiovascular risks (14, 15). Interestingly, contrary to reports of post-hyperthermia fatigue in other studies (14), our patients did not report significant weakness or malaise after WBH. This discrepancy may be attributable to the absence of general anesthesia in our protocol, allowing patients to recover more rapidly post-treatment.
The immunological effects of WBH warrant further investigation. Previous research has suggested that hyperthermia may stimulate immune responses by inducing the release of cytokines such as IL-1B, IL-6, IL-9, IL-10, and TNF-α (17, 19), potentially enhancing anti-tumor immunity. On the other hand, Hyperthermia enhances the immune response against tumors by increasing the expression of HSPs, which can activate DCs and promote T-cell responses. This is crucial for overcoming tumor-induced immune suppression .While our study did not specifically measure these parameters, the observed clinical responses suggest a possible immunological benefit that should be explored in future research. In summary hyperthermia's application in immunotherapy would provide valuable insights and highlight an innovative approach to enhancing cancer treatment outcomes
Therapeutic responses observed in our study were promising, with 80% of patients achieving CR, PR, or SD. The median time to progression (TTP) was approximately 18 weeks, comparable to findings from Hegewisch-Baker et al., who reported a TTP of 21 weeks in metastatic colorectal cancer patients (15). Additionally, Koga et al. reported PR and SD rates of 17.6% and 52.9%, respectively, in metastatic gastrointestinal cancer patients (14). Our results align well with these benchmarks.
Interestingly, we noted that patients who previously received chemotherapy regimens containing oxaliplatin or irinotecan responded favorably to repeat administration of these agents when combined with WBH. This observation is consistent with both in vitro and clinical studies indicating that hyperthermia enhances the cytotoxic effects of chemotherapy agents. For example, Raymond's in vitro study demonstrated a strong correlation between oxaliplatin concentration and hyperthermia duration in achieving cancer cell death (20).
One case of pulmonary embolism was observed during the study, but this was linked to the patient’s neutropenic fever and hospitalization rather than directly to the hyperthermia procedure. Consequently, our study did not find compelling evidence to support the routine use of anticoagulants during WBH sessions. However, clinicians should remain vigilant for thromboembolic complications, particularly in patients with other risk factors.
The most important limitation of this study was the small number of patients. Therefore, conducting research with a larger number of patients is essential. On the other hand, the patients included in these studies did not receive the same chemotherapy regimen and protocol, nor were they on the same treatment line. Consequently, another study with a sufficient number of patients and a relatively uniform chemotherapy regimen and protocol is necessary.
5.1. Conclusions
This study highlights the clinical potential of WBH as a supportive modality in the treatment of metastatic gastrointestinal cancers. The feasibility of performing WBH without general anesthesia marks a significant advancement, reducing procedural risks and promoting faster recovery times. Our findings suggest that WBH, when combined with standard chemotherapy protocols, can enhance therapeutic outcomes by improving drug efficacy and potentially overcoming resistance to previously administered chemotherapeutic agents. The absence of significant increases in chemotherapy-related toxicities in our study further underscores the safety profile of WBH. Cardiovascular monitoring remains essential during hyperthermia sessions to manage potential risks, especially in patients with preexisting cardiac conditions. The observed improvements in therapeutic response rates suggest that WBH may also stimulate immunological mechanisms, which warrants further exploration in future studies. Based on our findings, we recommend the following steps to advance the clinical application of WBH. Efforts should be made to incorporate WBH into national insurance policies to facilitate broader patient access. The cost-effectiveness of WBH, combined with its therapeutic benefits, supports its inclusion in routine oncological care.
Future research should involve larger sample sizes and randomized controlled trials to confirm our findings. Exploring the immunomodulatory effects of WBH and its role in overcoming chemotherapy resistance can provide valuable insights into optimizing cancer treatment protocols. Development of standardized protocols for WBH application, including patient selection criteria, monitoring protocols, and management of potential adverse events, will be crucial for integrating this modality into clinical practice. Finally, WBH combined with chemotherapy offers a promising therapeutic strategy for metastatic gastrointestinal cancer patients. With appropriate patient selection, continuous monitoring, and further research, this approach could significantly improve patient outcomes in oncology practice.