1. Background
Prostate cancer (PCa) is known as the second most common cancer among men. The mortality rate of this cancer increases with age, and its incidence rate is reported to be almost 60% in men older than 65 years (1, 2). Although various methods have been suggested to treat PCa, recurrence and progression of the disease are eventually observed in most patients (3). Additionally, the cost and side effects of some therapeutic approaches present challenges for patients (4). Several studies indicate that more than 80% of prostate cancers are androgen-dependent at early stages (5). The androgen signaling pathway plays an important role in the development and function of the prostate (6). Although endocrine therapy is designed to suppress the function of the androgen receptor (AR) by eliminating serum androgens, recurrence of PCa is observed in most patients after androgen deprivation therapy, leading to hormone-refractory PCa (7). The AR is a crucial factor in the development of both androgen-independent and androgen-dependent prostate cancers (8).
Active compounds from herbs have been shown to be effective in treating cancers without toxic effects (9). Among them, epigallocatechin gallate (EGCG) has high antioxidant potential (10). Various studies have evaluated the effect of EGCG on apoptosis induction in several cancer cell lines (11, 12). The results indicate that this polyphenol compound can increase apoptosis in cancer cells through different signaling pathways (13, 14).
Currently, new and effective approaches have been designed to use mesenchymal stem cells (MSCs) and active compounds from herbs in the treatment of different cancers (15, 16). Evidence shows that MSCs affect different signaling pathways in cancer cells and inhibit tumor cell growth (17, 18). However, reports exist about the pleiotropic effects of MSCs on cancer progression. Several studies show that these cells can influence cancer progression and suppression through pro-tumor and anti-tumor effects, respectively (19). These contradictory results may depend on the origin, dose, and duration of MSC treatment (20). Among different types of MSCs, human Wharton's jelly mesenchymal stem cells (WJ-MSCs) have several advantages for use in treating various disorders. These cells exhibit an immune-privileged status, and there are no ethical concerns in obtaining them (21-24).
2. Objectives
The present study was performed to investigate the potential of a combination of EGCG and conditioned medium derived from WJ-MSCs (WJCM) on the LNCaP cell line.
3. Methods
3.1. Cell Culture
In the present study, LNCaP cells and WJ-MSCs were obtained from the Pasteur Institute and the Cell Bank of Royan Institute, respectively. These cells were cultured in a medium containing DMEM-F12 and 10% fetal bovine serum (FBS).
3.2. Preparation of Conditional Medium Derived from Wharton's Jelly Mesenchymal Stem Cells
The WJ-MSCs were cultured until they reached approximately 80% confluence. Then, DMEM-F12 medium containing 0% FBS and 10% FBS was used to incubate these cells at 5% CO₂ and 37°C. After 24 hours, the medium was centrifuged at 4200 g for 20 minutes. The obtained supernatant was frozen at -20°C.
3.3. Preparation of Epigallocatechin Gallate
The stock solution of EGCG (Sigma-Aldrich, St. Louis, MO, USA) was prepared at a concentration of 0.1 M by dissolving it in dimethyl sulfoxide (DMSO).
3.4. MTT Assay
After seeding on 96-well plates, LNCaP cells were incubated with different concentrations of EGCG and conditioned medium derived from WJ-MSCs (WJCM) for 48 hours and 72 hours. Then, 20 µL of MTT (5 mg/mL) was added to each well. After incubation for 3 hours, the supernatant of the wells was removed, and 150 µL of DMSO was added. The optical density (OD) was measured at 570 nm using a microplate reader. Each concentration was tested in at least three replicates. The percentage of cell viability was calculated as control group OD/experimental group OD × 100.
3.5. Real-time PCR Analysis
The expression of apoptotic and AR pathway genes was evaluated in LNCaP cells treated with EGCG and WJCM, with at least a 30% reduction in cell viability observed in the MTT assay. For this purpose, LNCaP cells were cultured in 12-well plates. After 24 hours, these cells were treated with the determined concentrations of EGCG and WJCM. RNA was then extracted from the cultured cells using the RiboEx Total RNA extraction kit (GeneAll Biotechnology, Korea) and treated with DNase I (Thermo Fisher Scientific, Vilnius, Lithuania). cDNA synthesis was performed according to the instructions of the cDNA synthesis kit (Yekta Tajhiz Azma, Iran). The quantification of gene expression was determined by SYBR Green real-time PCR. The reaction was prepared from diluted cDNA, RealQ Plus Master Mix Green (Ampliqon, Denmark), and 0.1 pM of forward and reverse primers (Table 1) in a total volume of 25 µL. HSP90AB1 was used as the reference gene. Duplicate reactions were performed on a Rotor-Gene 6000 with preheating at 95°C for 10 minutes, followed by 45 cycles of denaturation at 95°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds. The specificity of the products was confirmed by melting curve analysis.
| Primer | Sequence (5´-3´) | Gen Bank Accession Number (Position) | Product Size (bp) |
|---|---|---|---|
| HSP90AB1 | NM_001271971.2; (exon 2/exon 3) | 157 | |
| R | GCGAATCTTGTCCAAGGCATCAG | ||
| F | GGAAGTGCACCATGGAGAGGA | ||
| BAX | NM_001291430.2; (exon 2/exon 3) | 195 | |
| R | TCACCCAACCACCCTGGTCTT | ||
| F | TGGCAGCTGACATGTTTTCTGAC | ||
| CASP3 | NM_001354783.2; (exon 8) | 100 | |
| R | ATTCTGTTGCCACCTTTCGG | ||
| F | TGGTTCATCCAGTCGCTTTG | ||
| CASP7 | NM_001227.5; (exon 7) | 108 | |
| R | TCCCCTTGGCTGTGTTTTG | ||
| F | GGAGAAAGCTCATGGCTGTGT | ||
| AR | NM_001424175.1; (exon 4/exon 6) | 168 | |
| R | GGACTTGTGCATGCGGTACTCA | ||
| F | CCTGGCTTCCGCAACTTACAC | ||
| PSA | NM_001648.2; (exon 3/exon 5) | 161 | |
| R | CCCCAGAATCACCCGAGCAG | ||
| F | ACCAGAGGAGTTCTTGACCCCAAA |
Primer Sequences Used for Expression Analysis in the Present Study
3.6. Statistical Analysis
The pffafl method was used to determine the relative expression of the studied genes. Data analysis was done using t-test. The P value < 0.05 was considered to be significant. The mean value was calculated from three independent experiments.
4. Results
4.1. Cell Viability Assessment
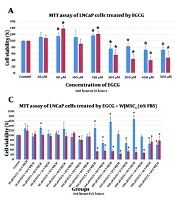
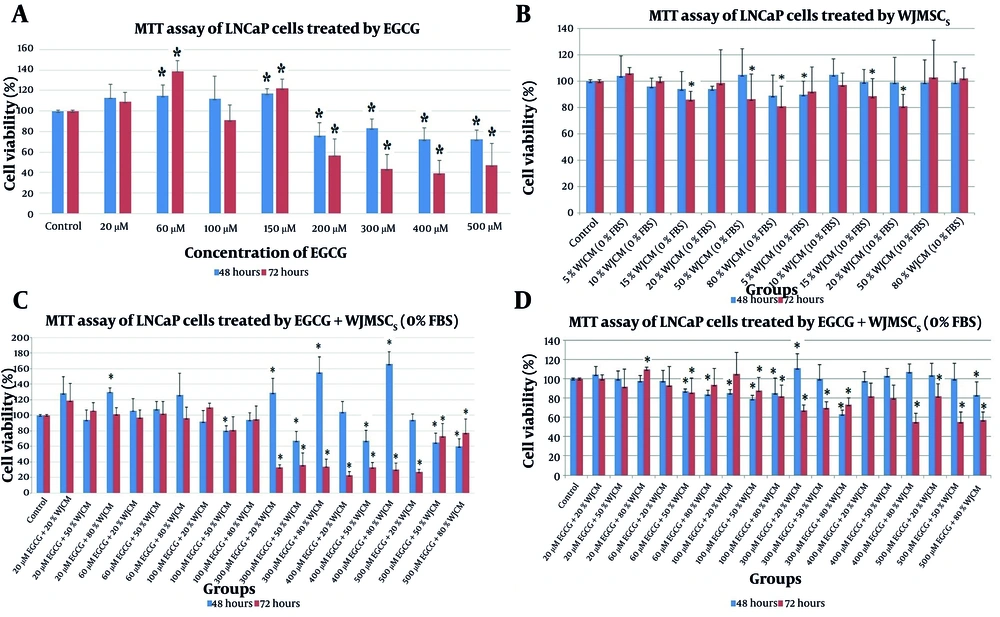
The results indicated that EGCG concentrations less than 200 µM had no noticeable effect on the viability of LNCaP cells. Cell viability decreased by approximately 30% in cells treated with 200 - 500 µM concentrations of EGCG for 72 hours (Figure 1A). The evaluation of cell proliferation assay results revealed no difference in the viability of LNCaP cells treated with WJCM compared with control cells (Figure 1B). Surprisingly, a decrease in viability of more than 30% was observed after treatment with different combinations of EGCG and WJCM for 48 hours and 72 hours (Figure 1C and D).
The results of cell proliferation assay following treatment of LNCaP cells with A, epigallocatechin gallate (EGCG); B, WJCM; C, EGCG+WJCM (0% FBS); and D, EGCG+WJCM (10% FBS) for 48 h and 72 h. Data were presented as mean of cell viability (%) ± SD (standard deviation) in the plus direction. * P < 0.05, WJCM; conditioned medium derived from Wharton's jelly mesenchymal stem cells (WJ-MSCs)
4.2. Real Time PCR Analysis
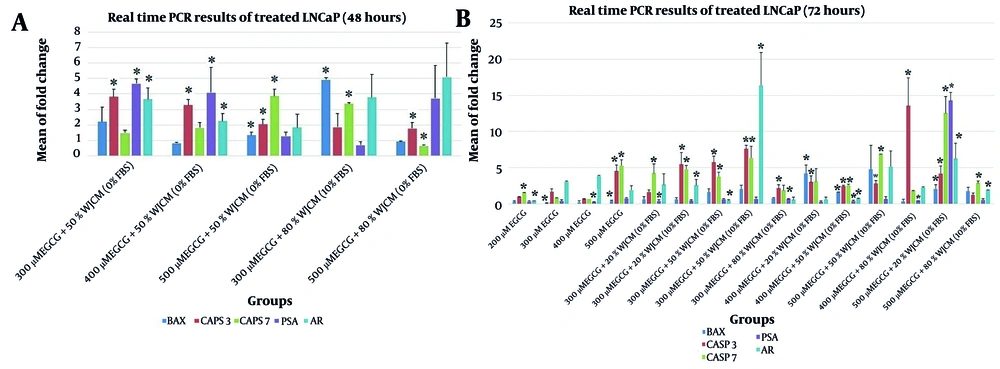
The expression of BAX, CASP3, and CASP7 genes significantly increased in LNCaP cells after treatment with 500 µM EGCG + 50% WJCM (0% FBS) for 48 hours, as well as 400 µM EGCG + 50% WJCM (0% FBS) and 500 µM EGCG + 20% WJCM (0% FBS) for 72 hours. Among these treatments, a significant decrease in the expression of AR and PSA genes was observed in LNCaP cells treated with 400 µM EGCG + 50% WJCM (0% FBS) for 72 hours (P < 0.05) (Figure 2).
The expression analysis of BAX, CASP3, CASP7, AR and PSA genes in the LNCaP cells treated by epigallocatechin gallate (EGCG) alone and its combination with WJCM for A, 48 h; and B, 72 h. Data was shown as mean of fold change ± SD (standard deviation). The mean fold of gene expression was defined to be 1 in the control cells (LNCaP cells treated by DMSO, data not shown). * P < 0.05; WJCM, conditioned medium derived from Wharton's jelly mesenchymal stem cells (WJ-MSCs)
5. Discussion
Various studies have been conducted to identify novel approaches for the treatment of PCa (25). In the present study, the effects of EGCG and WJCM were evaluated on the LNCaP cell line. The results from the proliferation assay indicated that treatment with high concentrations of EGCG (> 200 µM) for 72 hours significantly decreased the viability of LNCaP cells. The EGCG has been shown to reduce membrane fluidity through interaction with the LNCaP cell membrane (26). The EGCG exhibits anti-tumor activity by inhibiting proliferation, migration, and angiogenesis (27). Schroder et al. found that the viability of MCF-7 and MDA-MB-231 breast cancer cell lines decreased after treatment with EGCG concentrations greater than 45 µg/mL (28). However, the observed difference in minimum effective concentration may be explained by the different etiologies of breast and prostate cancers. Furthermore, the decreased viability of treated LNCaP cells was observed to be time-dependent at high EGCG concentrations (> 300 µM) (Figure 1A). These data are consistent with a previous study (26).
There are controversial reports about the role of MSCs in cancer treatment. Some researchers observed that treatment with MSCs was effective in cancer progression, while other studies indicated that these cells have the ability to suppress tumor growth (19). In the present study, the conditioned medium was prepared from WJ-MSCs. The conditioned medium has been demonstrated to contain various secreted growth factors (29). The results from the proliferation assay showed that WJCM had no effect on the viability of LNCaP cells. Previous evaluations of the effect of WJCM on tumor growth indicated that this medium has the potential to suppress growth in MDA-MB-231, TOV-112D, MG-63, HSC3, HepG2, PC3, SKOV3, and HeLa cancer cell lines (30). The inconsistency of the results obtained on LNCaP cells with others may be due to the distinct biological characteristics of different types of cancers (31).
Several studies have revealed that combination therapy is more effective than each agent alone. Adhami et al. evaluated the effect of EGCG and NS398 on cell lines and found that the combination of these two agents had a greater ability to decrease the viability of cancer cells compared with the effect of each agent alone (32). Lev-Ari et al. found similar results regarding the combination of curcumin and celecoxib on colorectal cancer cell lines (33). Consistent with previous studies, we found that the combination of EGCG and WJCM had a better effect on tumor suppression. In the present study, results from the proliferation assay showed that EGCG (300 - 500 µM) alone decreased the viability of LNCaP cells, but there was no significant increase in the expression of apoptosis genes in cells treated with these concentrations of EGCG. In contrast, the combination of EGCG and WJCM synergistically increased apoptosis in LNCaP cells compared with cells treated with EGCG alone.
The AR signaling pathway plays an important role in the progression and lethality of PCa (34). There is crosstalk between apoptotic and AR signaling pathways, and AR expression plays a dual role in the apoptosis of PCa cells. In some environmental conditions, AR positively induces apoptosis in PCa cells, while other studies indicate that downregulation of AR expression sensitizes PCa cells to apoptosis (35). Although treatment with EGCG (200 - 500 µM) for 72 hours was associated with decreased viability in LNCaP cells, expression analysis showed no upregulation of BAX, CASP3, and CASP7 genes and no downregulation of AR and PSA genes in the treated cells. However, 400 µM EGCG in combination with 50% WJCM (0% FBS) significantly increased the expression of apoptosis genes and decreased the expression of androgen signaling pathway genes in LNCaP cells, confirming the synergistic effect of combination therapy with EGCG and WJCM.
5.1. Conclusions
In the present study, our data suggest that the combination therapy of 400 µM EGCG and 50% WJCM (0% FBS) can suppress the growth of LNCaP cells. This combination strongly enhanced the expression of BAX, CASP3, and CASP7 genes and downregulated the expression of AR and PSA genes in LNCaP cells.